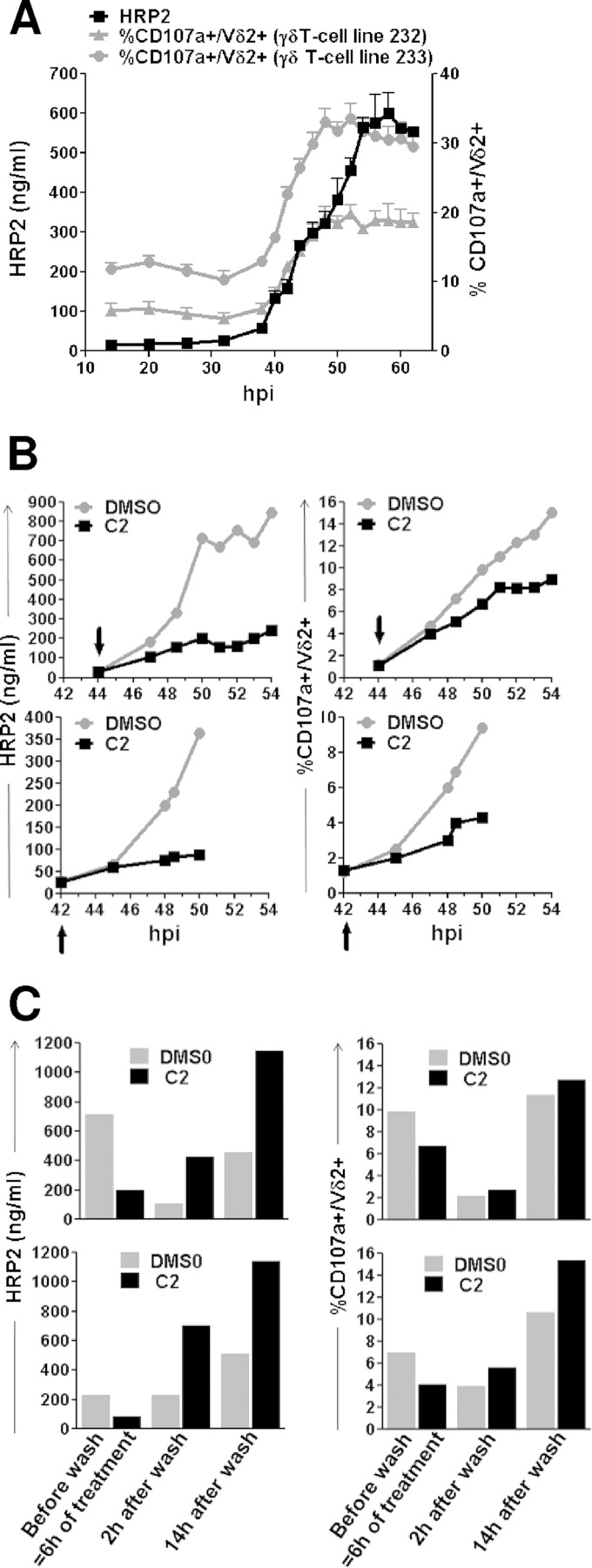
FIG 4.
Phosphoantigens are released during iRBC rupture. (A) Synchronized parasites from 3 independent cultures (A, B, and C) at 1% parasitemia were cultured in CPM for 62 h, and iRBC supernatants were collected at the indicated time points (hours postinvasion [hpi]) across the parasite developmental cycle (time zero corresponds to parasitic invasion). The phosphoantigen bioactivity in the various iRBC-SNs was assessed on two independent γδT-cell lines (232 and 233) using CD107a surface expression. HRP2 concentration in the iRBC-SNs was determined by ELISA. Data show the means ± SD from CD107a expression induced by the three independent culture SNs (A, B, and C) and their means ± SD for HRP2 content at each time point. (B) Synchronized iRBC cultures (1% parasitemia) were treated with compound 2 (C2) or a control (DMSO) at the time indicated by the arrow, and supernatants were collected at different time points of treatment. Two independent C2 treatment experiments are shown on 44-hpi schizonts (top) and 42-hpi schizonts (bottom). HRP2 content was measured by ELISA (left), and phosphoantigen concentrations were assessed by CD107a test (right) on 3 γδT-cell lines. Results for representative γδT-cell line 384 are shown. (C) Parasite cultures used for panel B were washed after 6 h of treatment with compound (C2), fresh medium was added, and supernatants were collected at the indicated times postwash and tested for both their HRP2 content (left) and their ability to induce Vγ9Vδ2 T-cell degranulation (right). Shown are results from one γδT-cell line (the same as that shown in panel B) out of three.