FIG 7.
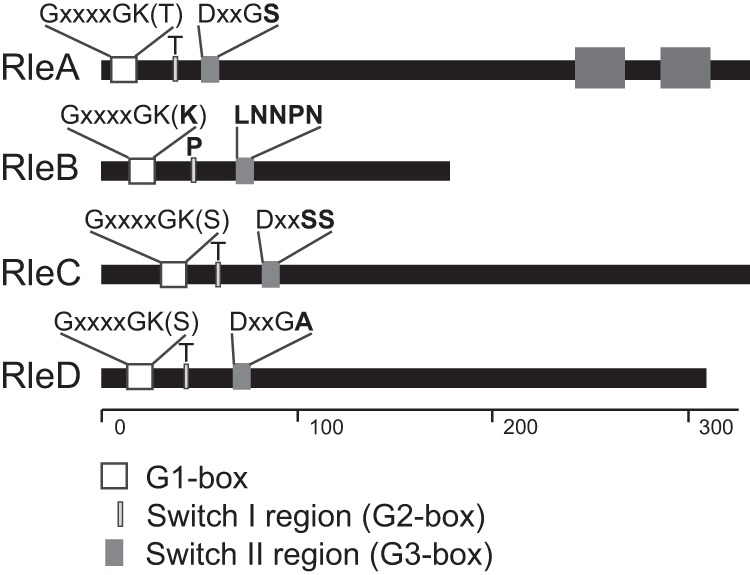
Rle proteins of L. longbeachae show various degrees of catalytic residue conservation. The figure shows a schematic representation of the L. longbeachae Rab-like effectors, with the catalytic residues highlighted. The scale along the bottom signifies the number of amino acids of each effector, highlighting that RleB is significantly smaller than the other effectors. RleA is predicted to carry two C-terminal transmembrane domains, represented by the dark gray boxes. The N-terminal white box represents the highly conserved G1 box with a consensus sequence of GXXXXGK(S/T), where X is any amino acid. The light gray box represents the switch I region which requires a conserved threonine (T) for function. The dark gray box represents the switch II region with a consensus sequence of DXXGL, where L is a hydrophobic residue. Residues that do not match the consensus sequence are shown in bold, demonstrating that RleB is unlikely to have GTPase activity.
