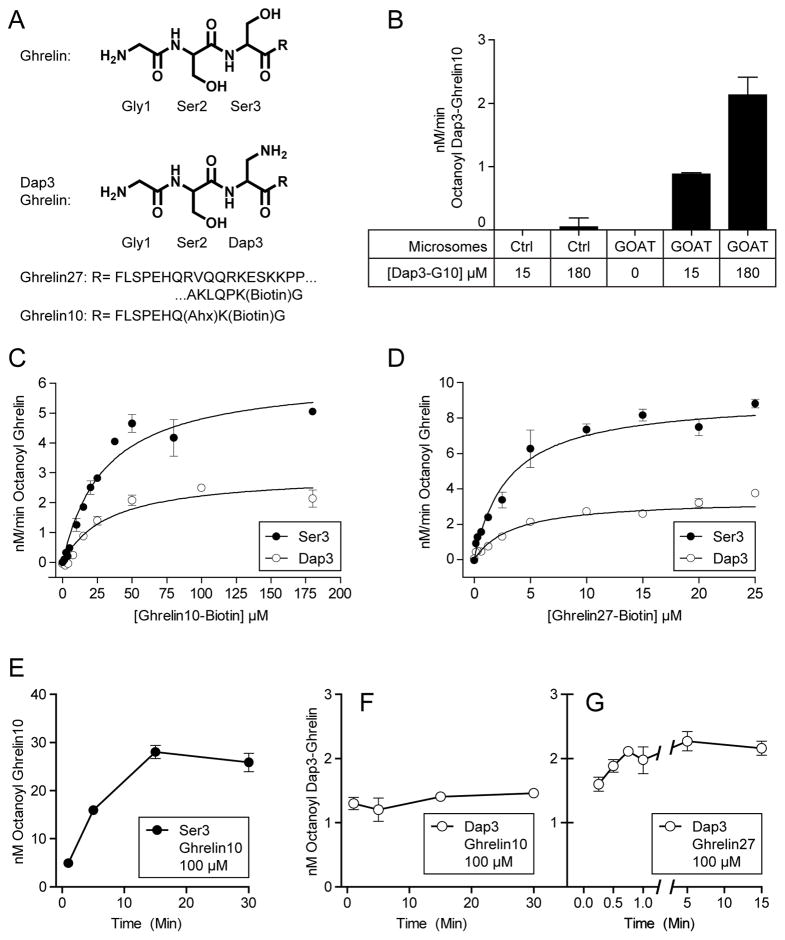
Figure 4. GOAT octanoylates Dap3 substrates.
(A) Structure of Ghrelin and Dap3 (amino-alanine) analog.
(B) Octanoylation of Dap3-Ghrelin10 (Dap3-G10) requires GOAT. 25 μg GOAT or empty-vector virus control microsomes were incubated for 1 min at 30°C with 1 μM octanoyl-CoA, 50 μM palmitoyl-CoA, and the indicated concentration of Dap3-Ghrelin10.
(C) Kinetic measurements for Ghrelin10 and Dap3-Ghrelin10 and D, kinetic measurements for Ghrelin27 and Dap3-Ghrelin27 with 1 μM octanoyl-CoA. Solid lines are best-fit to the Michaelis-Menten equation, and Km values are shown in Table 1.
(E) Octanoylation of 100 μM Ser3 Ghrelin10 over time. Each mixture contained 25 μg membrane protein, 1 μM octanoyl-CoA, and 50 μM palmitoyl-CoA.
(F,G) Octanoylation of Dap3-Ghrelin10 (100 μM) and Dap3-Ghrelin27 (10 μM) over time, respectively.