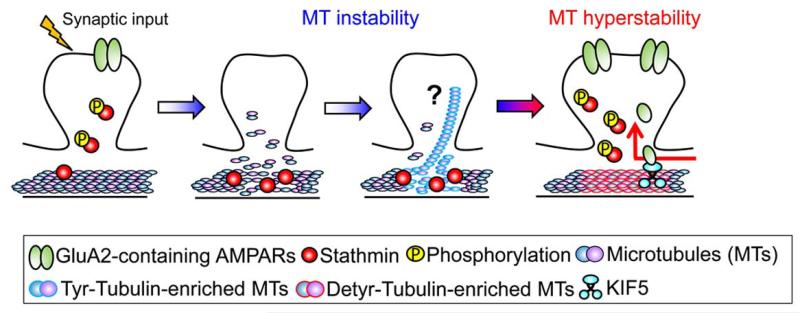
Figure 2. Proposed Model for the Role of Dynamic Microtubules in Memory Formation.
During the early phase (see Figure 2), microtubules become unstable due to the increased binding of stathmin to the tubulin caused by the hypophosphorylation of stathmin. Microtubule instability during the early phase is important for memory formation because treatment with microtubule stabilizer drug paclitaxel disrupts long-term memory formation (see text for details). During the late phase, phosphorylation of stathmin is increased and tubulin is dissociated from stathmin. Subsequently, the amount of detyrosinated-tubulin-enriched microtubules (stable microtubules) is increased. Tubulin detyrosination activates the transport of the GluA2 subunit from the soma to synaptic sites by enhancing the binding of the KIF5-GluA2 complex to microtubules. The microtubule hyperstability during the late phase is also important for memory, since treatment with microtubule stabilizer drug paclitaxel or microtubule destabilizer drug nocodazole 8 h following learning enhances or reduces long-term memory, respectively.