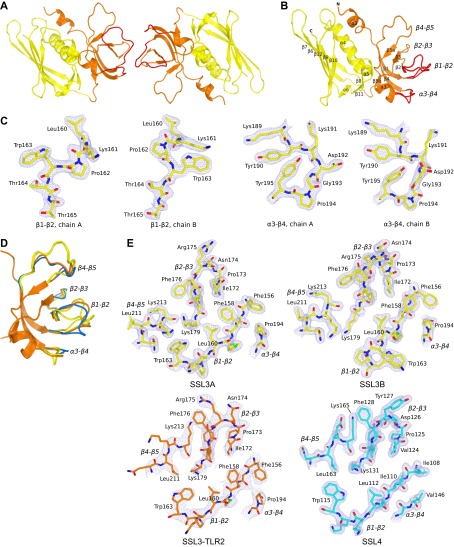
Fig. S2.
Structure of SSL3ΔN shows structural variation in loops of the β-grasp domain. (A) Cartoon visualization of the SSL3ΔN asymmetric unit that contains two molecules SSL3, both comprising residues 134–326. Two SSL3 molecules contact via three loops (β1–β2, β2–β3, and α3–β4) in the OB domain (orange), an arrangement that is clearly different from the previously observed SSL dimers formed by antiparallel packing of two β-grasp domains (yellow) (28). (B) Cartoon visualization of the SSL3ΔN structure showing the N-terminal OB domain (residues 134–227, orange) and the C-terminal β-grasp domain (residues 228–326, yellow). The OB domain comprises a five-stranded β-barrel and two short α-helices, and two conformations observed for the β1–β2 and α3–β4 loops are indicated in red. The β-grasp domain contains a central α-helix packed against a mixed five-stranded β-sheet. Residues in strand β10 and helix α5 contribute to a V-shaped binding site for sialyl LewisX containing glycans. (C) Two conformations of loop β1–β2 (panels 1 and 2) and loop α3–β4 (panels 3 and 4) that are observed in the crystal structure of SSL3 shown in stick representation in their corresponding 2Fo–Fc maps contoured at 1.2 σ above the average density. Except for the Lys191 side chain, these loops are well defined in the electron density maps, and differences likely arise from their involvement in crystal packing. (D) Structural variation of the β1–β2, α3–β4, and β4–β5 loops in superimposed structures of “free” SSL3 (SSL3A and B, yellow), SSL4 (blue; PDB ID code 4DXF) (38) and the SSL3–TLR2 complex (orange). Whereas free SSL3 differs substantially from TLR2-bound SSL3, the structure of SSL4 shows much smaller differences. (E) Structural variation of the TLR2 binding site as observed in the SSL3–TLR2 complex (orange; Lower Left) and the corresponding region in free SSL3A (yellow, Upper Left), SSL3B (yellow, Upper Right), and SSL4 (blue, Lower Right), all shown in stick representation in their corresponding 2Fo–Fc maps contoured at 1.2 σ above the average density. The orientation of the displayed regions is equal to Fig. 2C.