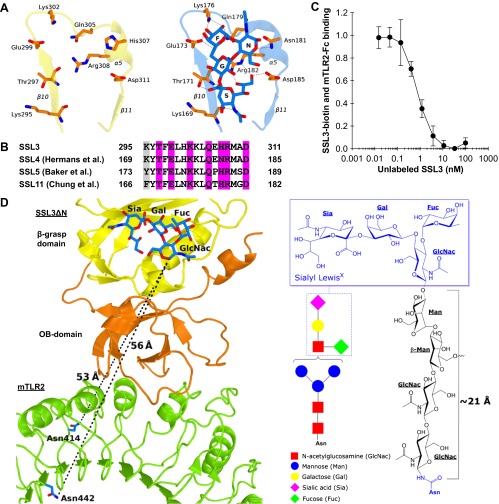
Fig. S3.
Nanomolar binding of SSL3 to TLR2 does not involve sialyl LewisX interactions. (A) Stick representation of residues that are observed to bind sialyl LewisX in SSL4 (Right) (PDB ID code 4DXG) (38) and the corresponding region of SSL3 (Left). Sialyl LewisX sugar moieties are labeled: sialic acid (S), galactose (G), N-acetyl glucosamine (N), and fucose (F). Hydrogen bonds between SSL4 residues and sialyl LewisX are indicated with dashed lines. (B) Sequence alignment of SSL3, SSL4, SSL5, and SSL11 shows the conserved sialyl LewisX binding pattern (TxExxKxxQx[N/H]RxxD; purple) (27, 28, 38). Residues highlighted in gray only contribute to the hydrogen bonding network through backbone atoms, and therefore these interactions are sequence independent. (C) SSL3 and TLR2 have nanomolar affinity. In an AlphaScreen competitive binding assay, unlabeled SSL3 was titrated in and competes with biotin-labeled SSL3 for TLR2-Fc binding. The IC50 value corresponds to a Kd of 0.6 ± 0.4 nM. Data are expressed relative to binding of SSL3–biotin to TLR2 with no unlabeled SSL3 present. Data points represent the mean ± SD of at least three independent experiments. (D) Geometric analysis of the feasibility of sialyl LewisX interactions in the SSL3–TLR2 complex. (Left) Sialyl LewisX was positioned in the SSL3–TLR2 complex by superimposing the LewisX binding sites of SSL3 and the SSL4–sialyl LewisX complex (PDB ID code 4DXG) (38). Indicated are the shortest distance between the LewisX glycan and the two nearest glycosylated asparagine residues in TLR2 (shown in blue sticks). (Right) Chemical structure of a N-linked glycan including a mannose-linked sialyl LewisX [GlcNac(-Fuc)-Gal-Sia]. The linker between the asparagine and LewisX consists of two N-acetylglucosamine and two mannose units that can span a distance of at most 21 Å in a fully extended conformation, as was estimated on the basis of crystal structures in the protein data bank that contain this glycan motif. This distance is twofold smaller than the distances to the nearest N-linked asparagines in TLR2, excluding the involvement of sialyl LewisX binding in the observed SSL3–TLR2 complex.