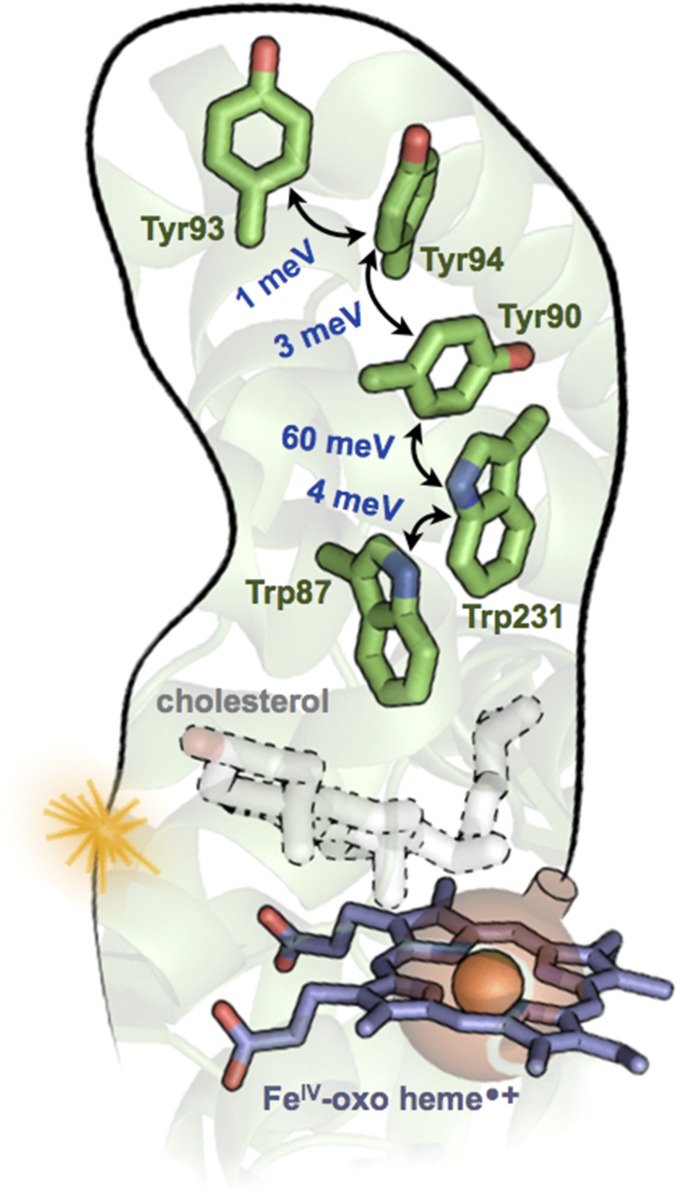
Fig. 2.
A chain of Trp and Tyr residues in the cytochrome P450 protein CYP11A1 (PDB ID code 3N9Y) discussed by Gray and Winkler (1). DFT-derived V values are shown in blue [the method in refs. 6 and 7 was applied using NWChem (8), with the M06-HF functional, the D3 dispersion correction, the cc-pVTZ basis set for C and H atoms, and aug-cc-pVTZ for N and O]. Each hopping rate is proportional to V2. The greater than 10-fold stronger V between Tyr90–Trp231 arises from its PD geometry, compared with the T geometry of the other pairs. Because each pair of residues is within vdW contact, no protein medium is included in the computations of V. The Fe(IV)-oxo heme radical cation (CpdI) of P450, shown as a bomb ready to explode, converts cholesterol to pregnenolone. If unable to react with cholesterol, CpdI might be defused by the nearby Trp87 (followed by hole hopping to the protein surface at Tyr93) before damaging the protein.