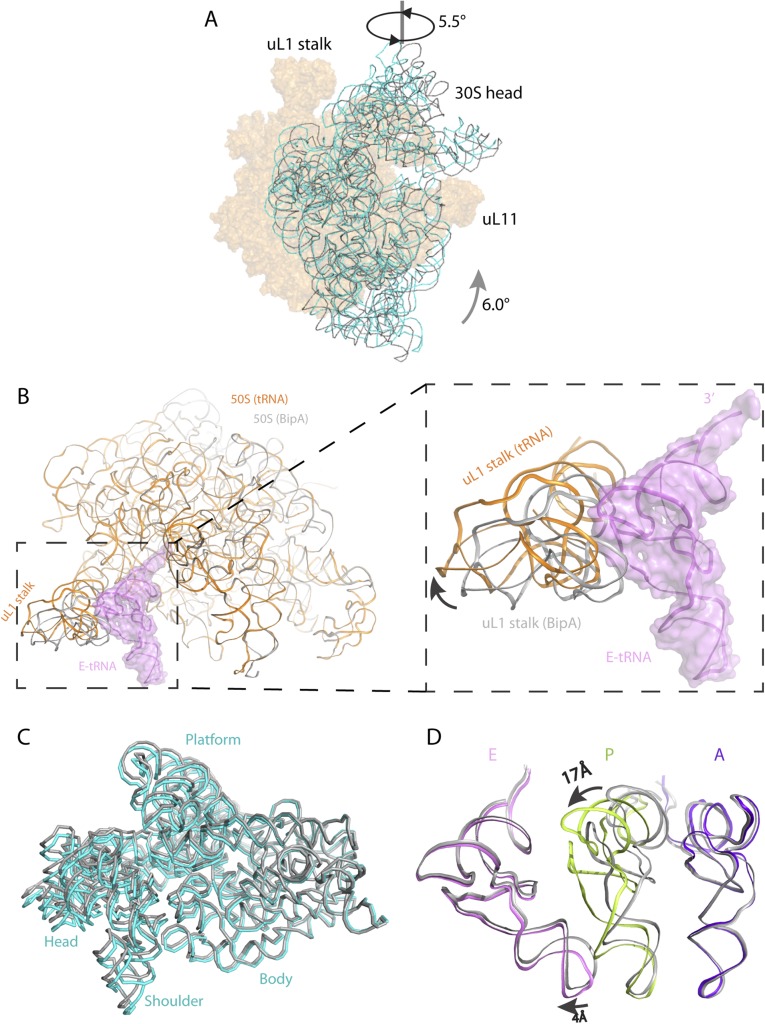
Fig. S6.
Comparison of the BipA–ribosome complex with the POST complex (EF-G bound) and with the BipA–tRNA–ribosome complex. (A) Head swiveling and body rotation of the 30S subunit relative to the 50S subunit in the BipA–ribosome complex. The 16S rRNA (cyan) shows a counterclockwise rotation by 6° of the 30S body and an orthogonal swiveling by 5.5° of the 30S head toward the uL1 stalk (viewed from the solvent side of the 30S subunit), compared with 16S rRNA (gray) in the POST complex (EF-G bound to the posttranslocational ribosome in canonical state trapped by fusidic acid) (14). The two structures are aligned on 23S rRNA. (B) The uL1 stalk in 50S of tRNA/mRNA ribosome complex (orange) moves away from the position of E-site tRNA (colored violet with surface), resulting in an open form. The 50S and uL1 stalk in the BipA–ribosome complex is colored gray. (C) Comparison of 30S subunits. The body of 16S rRNA of the BipA–tRNA–ribosome complex is almost identical to that without tRNA, whereas the head swivels less, with a ∼2° of 30S head swiveling in the former complex and a ∼5.5° in the latter, respectively. The 16S rRNA in the BipA–tRNA–ribosome complex and the BipA–ribosome complex are colored cyan and gray, respectively. (D) Comparison of A-, P-, and E-site tRNAs in our structure with that in classical state (34). The A- and E- site tRNAs are in a similar positioning in ribosome, despite that a 4Å shift of anticodon loop was observed in E-site tRNA following 30S head swiveling. A large shift (∼17 Å) was observed in the TΨC loop compared our structure with that in the classical P-site tRNA. The A-, P-, and E-site tRNAs in current structure are colored purple blue, limon, and violet, respectively, whereas those of the classical state are colored gray.