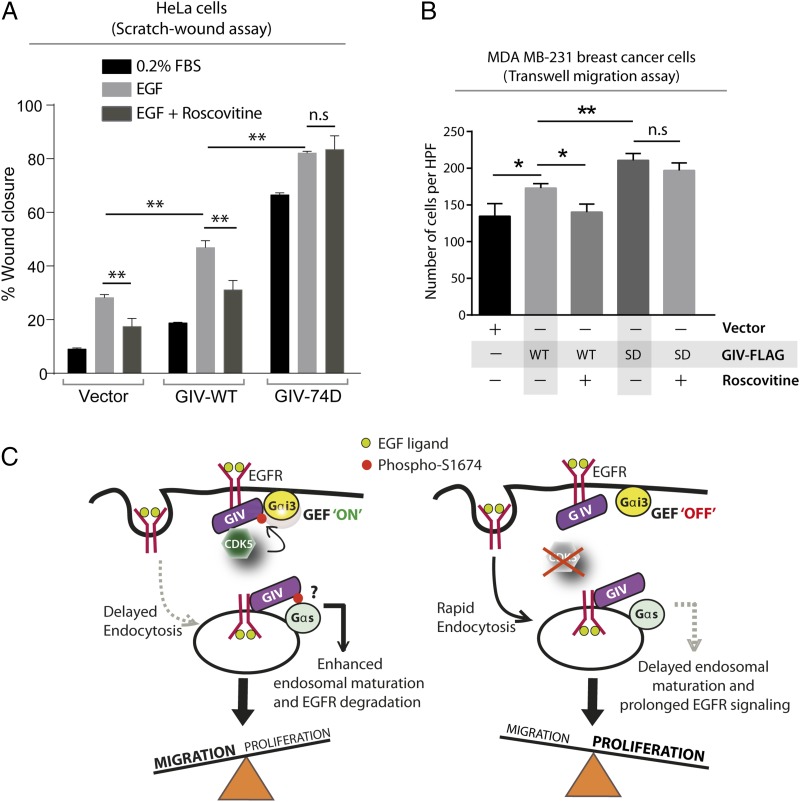
Fig. 5.
Cell migration after scratch-wounding and cancer cell chemotaxis were inhibited by Roscovitine in cells that express GIV-WT, but not in those expressing the phosphomimic 74D GIV mutant. (A) GIV-depleted HeLa cell lines stably expressing vector control, GIV-WT, and GIV-74D were treated or not with EGF in the presence or absence of Roscovitine and analyzed for cell migration after scratch wounding as in Fig. 2C. Bar graph shows the migration index (wound closure) of each cell line under each condition. Results are expressed as mean ± SEM; n = 3. (B) MDA-MB231 cells were evaluated for chemotaxis toward EGF as in Fig. 3B in the presence (+) or absence (−) of Roscovitine. The number of migrating cells was averaged from 20 field-of-view images per experiment. Results are expressed as mean ± SEM; n = 3; n.s., not significant. (C) Schematic of a working model. (Left) EGF stimulation activates CDK5, which turns GIV’s GEF activity “on” by phosphorylating it at S1674. This phosphoevent enhances GIV's ability to bind and activate Gαi at the PM, which in turn prolongs motogenic EGF signaling at the PM, likely via a delay in endocytosis. Such phosphorylation also enhances GIV's ability to bind Gαs (and perhaps modulate its activation; “?”) on endosomes, which speeds up endosomal maturation and suppresses mitogenic EGF signaling from endosomes. Overall, phosphorylation of GIV by CDK5 at S1674 triggers preferential cell migration over proliferation. (Right) Inhibition or depletion of CDK5 maintains GIV's GEF function in the “off” state, reducing GIV's ability to bind and modulate Gαi/s at both locations. Consequently, EGF triggers an opposite response, such that inhibition of CDK5 favors proliferation over cell migration.