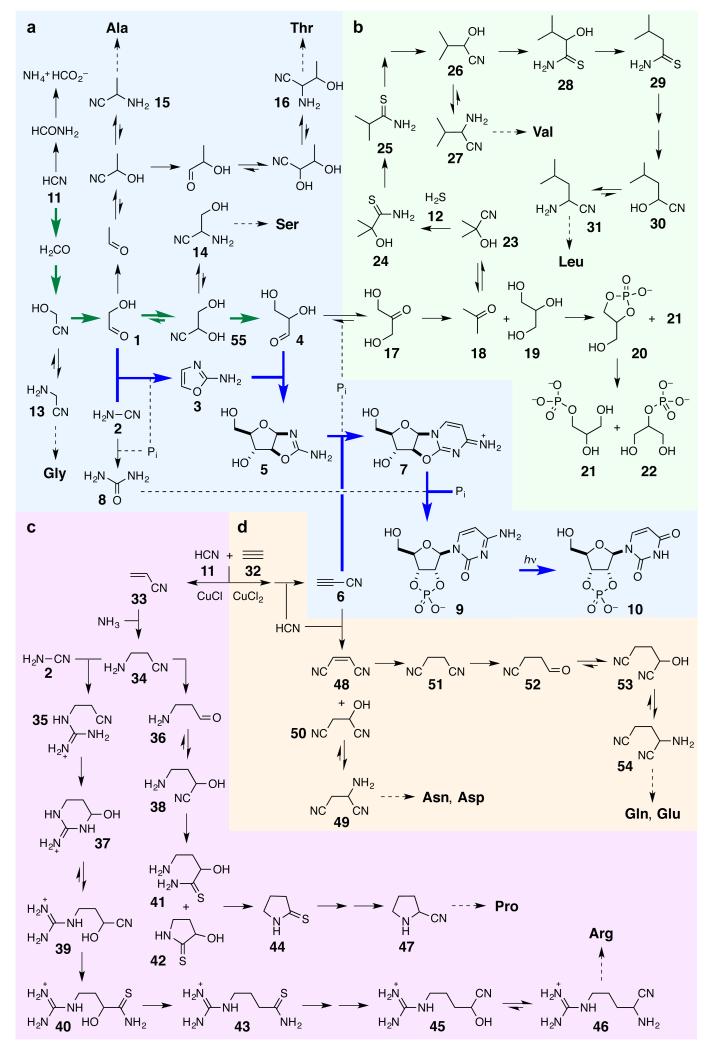
Figure 1. Reaction network leading to RNA, protein and lipid precursors.
The degree to which syntheses of ribonucleotides and amino acid and lipid precursors are interconnected is apparent in this ‘big picture’. The network does not produce a plethora of other compounds, however, suggesting that biology did not select all of its building blocks, but was simply presented with a specific set as a consequence of the (photo)chemistry of hydrogen cyanide 11 and hydrogen sulfide 12, and that set turned out to work. To facilitate description of the chemistry in the text, the picture is divided into four parts. a. Reductive homologation of hydrogen cyanide 11 (bold green arrows) provides the C2 and C3 sugars – glycolaldehyde 1 and glyceraldehyde 4 – needed for subsequent ribonucleotide assembly (bold blue arrows), but also leads to precursors of Gly, Ala, Ser and Thr. b. Reduction of dihydroxyacetone 17 – the more stable isomer of glyceraldehyde 4 – gives two major products acetone 18 and glycerol 19. Reductive homologation of acetone 18 leads to precursors of Val and Leu whilst phosphorylation of glycerol 19 leads to the lipid precursor glycerol-1-phosphate 21. c. Copper(I) catalysed cross-coupling of hydrogen cyanide 11 and acetylene 32 gives acrylonitrile 33, reductive homologation of which gives precursors of Pro and Arg. d. Copper(II) driven oxidative cross-coupling of hydrogen cyanide 11 and acetylene 32 gives cyanoacetylene 6 which serves as a precursor to Asn, Asp, Gln and Glu.