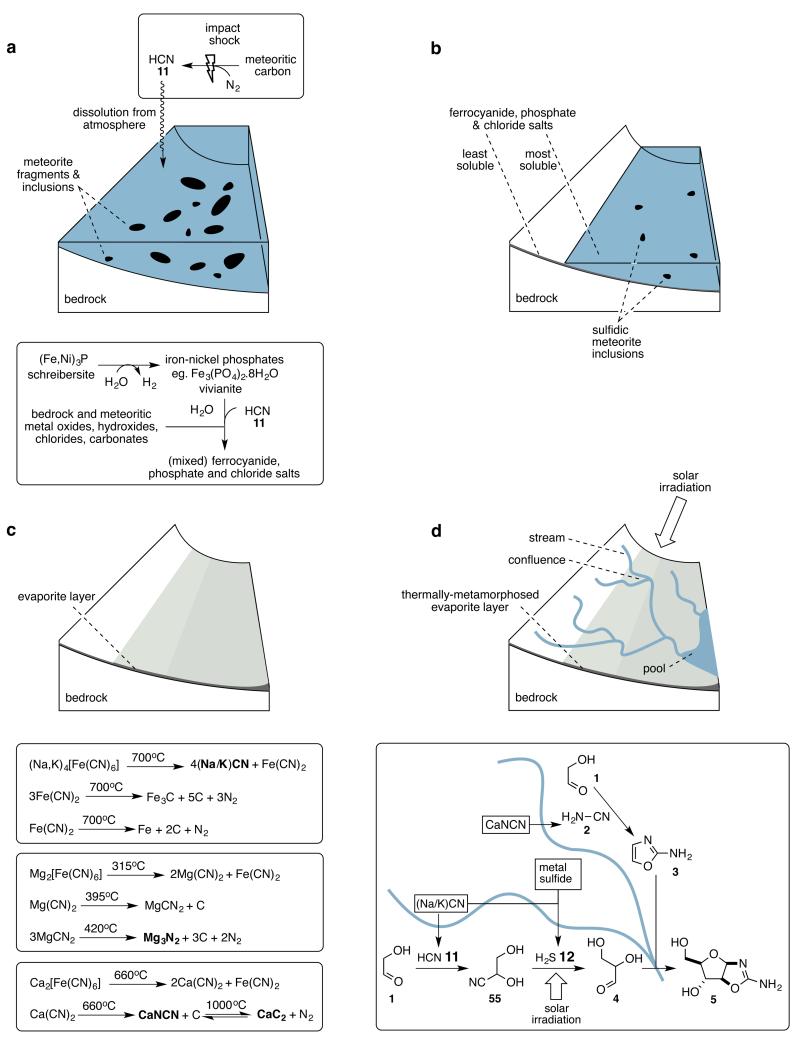
Figure 2. Chemistry in a post meteoritic impact scenario.
A series of post impact environmental events are shown along with chemistry (boxed) proposed to occur as a consequence of those events.
a. Dissolution of atmospherically produced hydrogen cyanide results in conversion of vivianite – the anoxic corrosion product of the meteoritic inclusion schreibersite – into mixed ferrocyanide salts and phosphate salts, counter cations being provided through neutralisation and ion-exchange reactions with bedrock and other meteoritic oxides and salts.
b. Partial evaporation results in the deposition of the least soluble salts over a wide area, further evaporation deposits the most soluble salts in smaller, lower lying areas.
c. After complete evaporation, impact or geothermal heating results in thermal metamorphosis of the evaporite layer, and generation of feedstock precursor salts.
d. Upper. Rainfall on higher ground leads to rivulets or streams that flow downhill sequentially leaching feedstocks from the thermally metamorphosed evaporite layer. Solar irradiation drives photoredox chemistry in the streams. Lower. Convergent synthesis can result when streams with different reaction histories merge, as illustrated here for the potential synthesis of arabinose aminooxazoline 5 at the confluence of two streams that contained glycolaldehyde 1, and leached different feedstocks before merging.