Fig. 4.
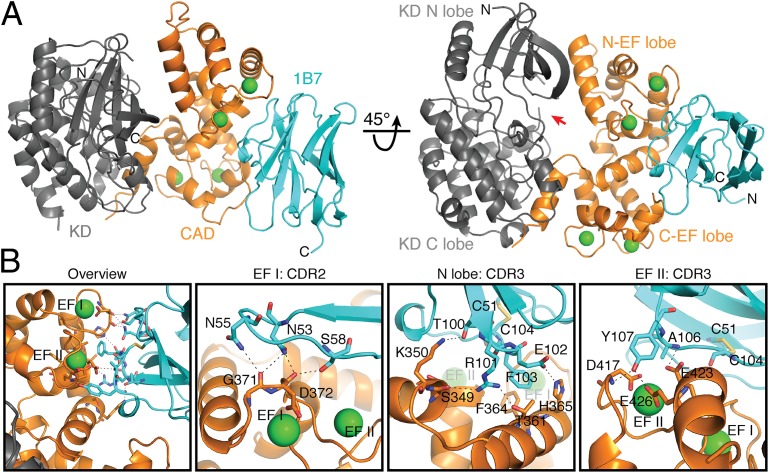
1B7 stabilizes a novel conformation of TgCDPK1. (A) Cartoon depicting the structure of the complex with the catalytic KD (gray), the CaM-like CAD (orange), bound calcium ions (green spheres), and 1B7 (blue). The position of the active site pocket is indicated (red arrow). (B) Specific interactions within the CAD:1B7 binding interface. Hydrogen bonds and salt bridges are depicted as black dotted lines. Interacting residues are shown as sticks for the CAD and 1B7. Cys51, in the beta strand immediately preceding CDR2, and Cys104, in CDR3, form a stabilizing, intrachain disulfide in 1B7. CDR1 on 1B7 makes no contacts with TgCDPK1.