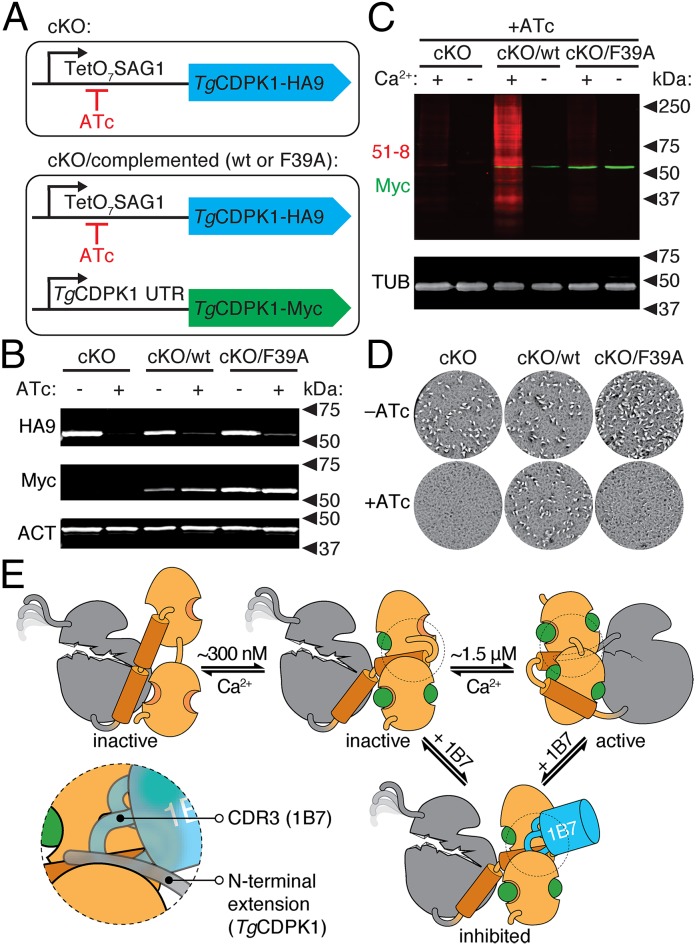
Fig. 7.
Allosteric activation is required for TgCDPK1 activity in vivo. (A) Strategy depicting the TgCDPK1 cKO carrying an HA9-tagged allele that can be shut down by addition of ATc. The complemented strains additionally carry a constitutive Myc-tagged allele (WT or F39A), expressed under the endogenous TgCDPK1 promoter. (B) Immunoblot of regulatable (HA9) or constitutive (Myc) TgCDPK1 alleles in the different strains grown in the presence or absence of ATc for 48 h. Parasite actin (ACT) is included as a loading control. (C) TgCDPK1-dependent thiophosphorylation (51-8; red) in lysates from the various parasite strains following growth in the presence of ATc. Reactions were performed in the presence or absence of Ca2+. Tubulin is included as a loading control. (D) Plaque formation by the various strains in the presence or absence of ATc. (E) Model for the activation of TgCDPK1 and its inhibition by 1B7. The KD (gray) is intrinsically inactive and its catalytic site is occluded by the CAD (orange). At ∼300 nM Ca2+, the CAD is partially occupied by Ca2+ and can bind 1B7 (blue). Higher Ca2+ concentrations are required for full Ca2+ occupancy of the CAD and stabilization of the KD, which leads to activation. The region of the CAD competitively bound by 1B7 and the N-terminal extension is circled and highlighted in the Inset.