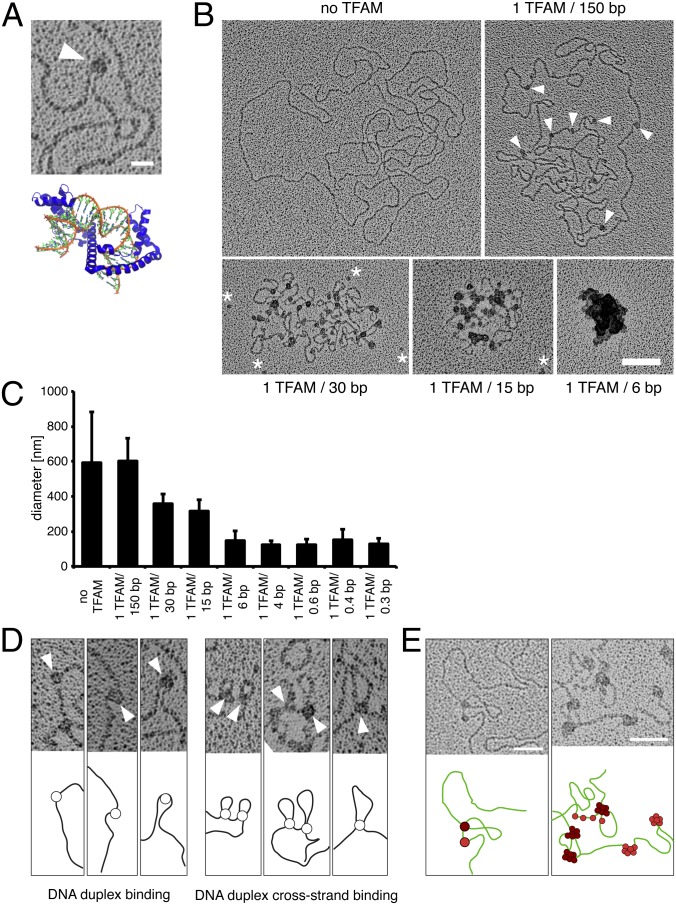
Fig. 1.
Electron microscopy reveals that TFAM packages single mtDNA molecules and that cross-strand TFAM binding is necessary for compaction of mtDNA. (A) Electron micrograph showing a DNA U-turn with TFAM bound (white arrowhead). (Scale bar: 20 nm.) Crystal structure of TFAM (blue) and DNA (orange/green) [Protein Data Bank ID code 3TMM (16)]. (B) Electron micrographs of spread DNA incubated with increasing concentrations of TFAM. TFAM molecules are indicated by white arrowheads. White asterisks mark unbound TFAM molecules. (Scale bar: 100 nm.) (C) Diameters of DNA incubated with increasing concentrations of recombinant TFAM protein by the mean of the long and short axes. Data are represented as mean ± SD, n = 134. (D) TFAM binds to DNA in two different ways. TFAM binds single DNA duplexes as beads on a string inducing bending of DNA (Left) or bridges two DNA duplexes resulting in loops (Right). TFAM molecules are indicated by white arrowheads. (E) TFAM binding to DNA as beads on a string (red) or by bridging two DNA duplexes (dark red). Subsequent binding preferentially occurs at sites already occupied by TFAM as can be observed by an increase in particle size. (Scale bars: 50 nm.)