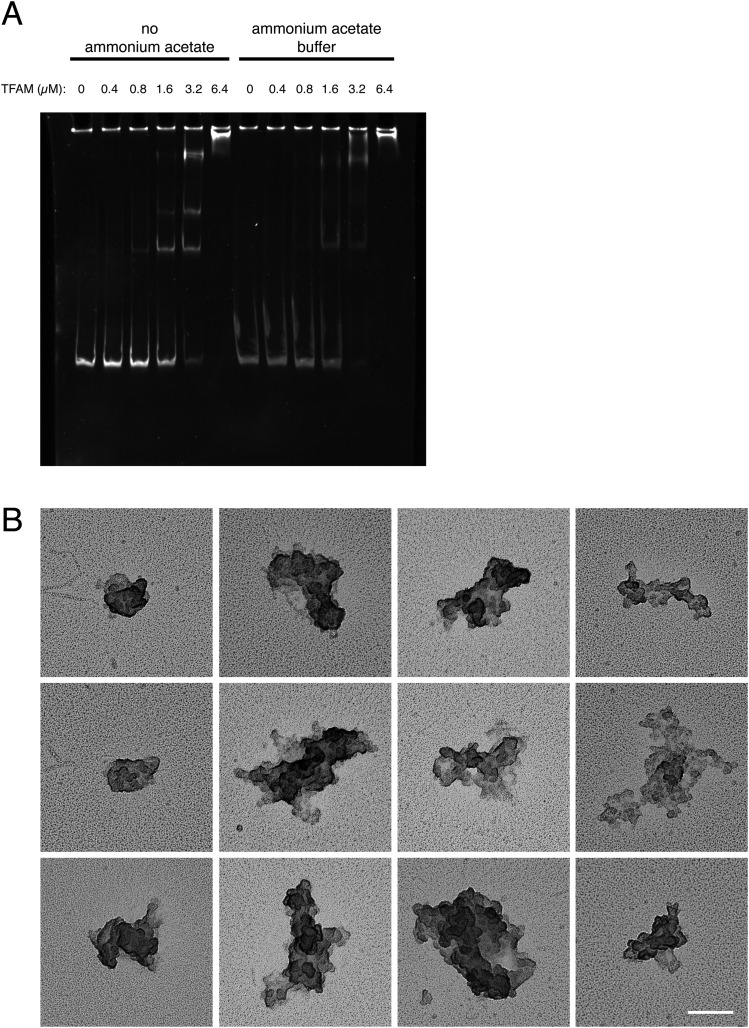
Fig. S1.
(A) EMSA of DNA with increasing concentrations of recombinant TFAM. The stability of the formed complex was investigated with or without ammonium acetate in the buffer. Constant amounts of DNA templates were incubated with increasing amounts of recombinant TFAM protein in standard and ammonium acetate buffer. A clear shift of the DNA fragments was visible in the EMSA, indicating that the ammonium acetate required for EM does not interfere with TFAM–DNA binding. (B) Representative electron micrographs of in vitro-generated nucleoids with TFAM concentrations >1 TFAM/6 bp. (Scale bar: 100 nm.)