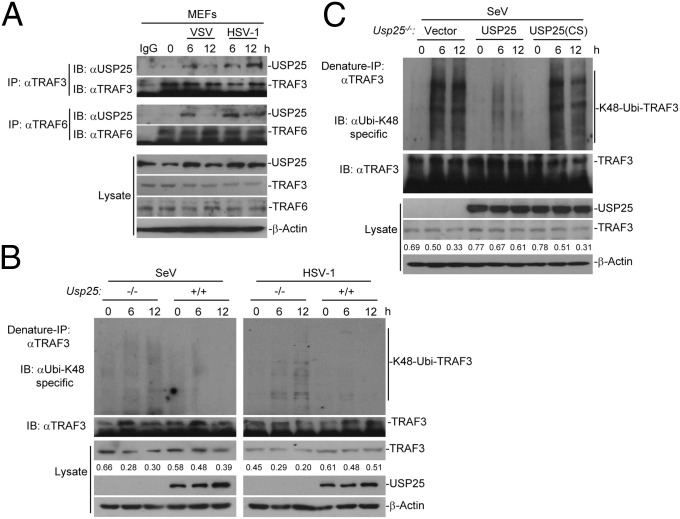
Fig. 5.
USP25 interacts with TRAF6 and TRAF3 after virus infection. (A) USP25 interacted with TRAF3 or TRAF6 after SeV or HSV-1 infection in primary MEFs. MEFs were infected with SeV or HSV-1 for the indicated time points. Cells were lysed and the cell lysates were immunoprecipitated with anti-TRAF3 or anti-TRAF6. The immunoprecipitates were analyzed by immunoblot with anti-USP25, anti-TRAF3, or anti-TRAF6 (top four panels). The expression levels of the proteins in the lysates were analyzed by immunoblot with the indicated antibodies (bottom four panels). (B) K48-linked ubiquitination of TRAF3 was enhanced in Usp25−/− BMDCs after SeV or HSV-1 infection compared with the wild-type BMDCs. Wild-type and Usp25−/− BMDCs were infected with SeV or HSV-1for the indicated time points followed by denature immunoprecipitation (Denature-IP) and immunoblot assays with the indicated antibodies. (C) SeV-induced K48-linked ubiquitination of TRAF3 was inhibited in Usp25−/− MEFs reconstituted with USP25 but not those with USP25(C178S). Usp25−/− MEFs were reconstituted with empty vector, USP25 or USP25(C178S) and infected with SeV followed by Denature-IP and immunoblot assays with the indicated antibodies. Data are representative of three independent experiments.