FIGURE 3.
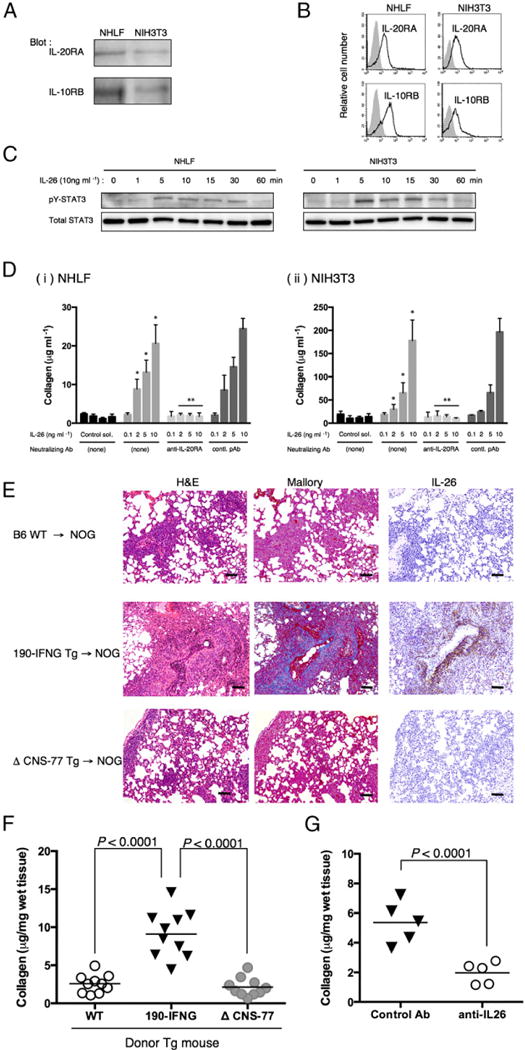
IL-26 stimulates fibroblast, and collagen deposition in the lung of obliterative bronchiolitis is induced in NOG mice receiving bone marrow cells and splenocytes of IL26 Tg mice. (A) Western blot analysis of IL-20RA and IL-10RB in NHLF and NIH3T3 cells. Lysates (each, 10 μg) were prepared using RIPA buffer, being resolved by SDS-PAGE in reducing condition and immunoblotted using anti–IL-20RA or anti–IL-10RB pAbs recognizing both human and murine Ags. (B) Representative histogram of IL-20RA and IL-10RB of NHLF and NIH3T3 cells. Single-suspension cells were stained with anti–IL-20RA or anti–IL-10RB pAbs recognizing both human and murine Ags, and analyzed using flow cytometry (bold line). Gray histograms indicate isotype control (rabbit polyclonal IgG and anti-rabbit IgG-PE). (C) A total of 1 × 105 cells of NHLF or NIH3T3 was seeded in 96-well flat-bottom plate, and the next day IL-26 (10 ng ml−1 in PBS) was added to each well. Cells were harvested at each time point, and cell lysates were prepared in RIPA buffer containing Halt Protease and Phosphatase inhibitor mixture, and 10 μg each lysate was resolved by SDS-PAGE in reducing condition and immunoblotted with antiphosphorylated STAT3 (pY-STAT3) recognizing both human and murine Ags, followed by stripping and reprobing with anti-STAT3 pAb (total STAT3) recognizing both human and murine Ags. Representative data are shown from three independent experiments with similar results. (D) Collagen production of NHLF (Di) or NIH3T3 (Dii) cells stimulated with exogenous IL-26 in the presence or absence of neutralizing anti–IL-20RA pAb or rabbit Ig (contl. pAb). Data are shown as mean ± SEM, resulting from three independent experiments with triplicates. The amount of secreted soluble collagen increased with increasing level of exogenous IL-26 in a dose-dependent manner (*p < 0.0001 versus corresponding control solvent), and production of collagen was inhibited by the presence of neutralizing anti–IL-20RA pAb (**p < 0.0001 versus corresponding contl. pAb). (E) H&E, Azan-Mallory staining, and anti–IL-26 immunohistochemical staining of sequential sections of the lung from NOG mice at 4 wk after transplantation of BM and splenocytes isolated from parental B6 (B6 WT), 190-IFNG BAC Tg (190-IFNG Tg), or ΔCNS-77 Tg mice. The lung of recipients of 190-IFNG Tg mice showed obliterative bronchiolitis with collagen deposition and IL-26+ cell infiltration, whereas recipients of B6 WT or ΔCNS-77 Tg mice showed peribronchial and septal infiltration without collagen deposition or IL-26+ cells. Representative histology is shown from three independent experiments (for each, n = 6). Original magnification ×100. Scale bars, 100 μm. (F) Recipient mice were sacrificed at 4 wk posttransplantation, and the lungs were removed. Collagen contents in the lung were quantified by Sircol Collagen Assay. The mean number (±SEM) of total collagen contents (μg) per wet lung tissue weight (mg) was determined from three independent experiments (for each, n = 10). Increased collagen contents were clearly observed in recipients of 190-IFNG Tg mice, compared with those in recipients of B6 WT or ΔCNS-77 Tg mice. Each dot indicates individual value, and horizontal bars indicate mean value. (G) Whole CB transplant mice were administered anti–IL-26 Ab or control Ab (each 50 μg/dose) i.p. thrice per week, beginning at day +21 after transplantation until day +32. Recipient mice were sacrificed at 5 wk posttransplantation, and the lungs were removed. Collagen contents in the lung were quantified by Sircol Collagen Assay. The mean number (±SEM) of total collagen contents (μg) per wet lung tissue weight (mg) was determined from two independent experiments (for each, n = 5). Decreased collagen contents were clearly observed in the recipients of anti–IL-26 Ab. Each dot indicates individual value, and horizontal bars indicate mean value.
