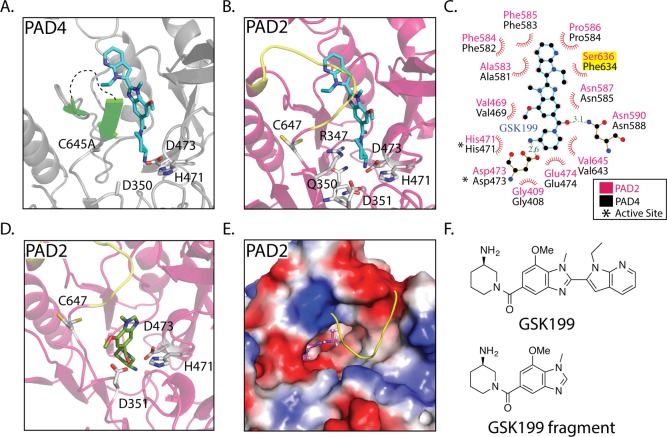
Figure 4.
Allosteric inhibitors targeting the PADs. (A) Crystal structure of PAD4 bound to GSK199 as seen in Lewis et al. Flexible loop that interacts with GSK199 is depicted in green. Unstructured loop containing residues 639–640 is shown as a black dashed line. (B) Alignment of the PAD4 GSK199 and PAD2·Ca(10mM)2+ crystal structures, followed by removal of the PAD4 structure to depict where GSK199 could bind in PAD2·Ca(10mM)2+. The PAD2·Ca(10mM)2+ loop (yellow) consisting of residues 638–645 would have to move to accommodate GSK199. This is probable because this loop takes on a more flexible form in the holoPAD2 structure. (C) Ligplot+ representation of GSK199 interacting with PAD4 in the crystal structure, with residues from PAD4 labeled in black and the corresponding residues from PAD2 in pink. (D) Docking of a fragment of GSK199 in the PAD2·Ca(10mM)2+ structure reveals a tight binding pocket and potential inhibitor for PAD2. (E) Surface representation and top down view of the docked GSK199 fragment. (F) Inhibitor structures found in panels a–e.