Abstract
The pathogenesis of renal artery aneurysms (RAAs) is degenerative, which eventually leads to weakening of the vessel wall and, in extreme cases, rupture. RAAs are a rare occurrence. Patients generally are asymptomatic, with a small number presenting with uncontrollable hypertension or hematuria. Most RAAs are discovered incidentally on imaging and do not pose an immediate health threat. However, the risk of rupture is an indication for prophylactic repair in certain patients. Interest in interventional radiologic procedures in the management of RAAs has recently increased; however, open repair should still be considered in select instances. In this case series, we present three patients for whom an open approach was indicated and performed.
Renal artery aneurysms (RAAs) are a rare occurrence, with an incidence of about 1% (1, 2). Most patients are asymptomatic and are diagnosed through incidental findings on imaging. In rare instances, the aneurysm can rupture and cause significant morbidity and mortality. Patients with risk factors of RAA rupture, such as pregnancy, polyarteritis nodosa, or large aneurysm size, should be considered for elective repair (3, 4). Endovascular therapy has gained recognition in the management of RAAs; however, only a subset of patients fit the anatomic criteria to undergo this therapy, and the remainder require surgical treatment of the RAA. We reviewed the records of three patients with RAAs who were treated at Baylor Jack and Jane Hamilton Heart and Vascular Hospital from July 2014 to October 2014 with open repair.
CLINICAL CASES
All patients were asymptomatic and had their RAA discovered during routine imaging (case 1 for hepatitis C–related cirrhosis Child-Pugh Class A; case 2 for liver transplant surveillance; case 3 for workup of elevated liver function tests). The clinical information for these patients is summarized in Table 1. In each case, the RAA involved the renal artery bifurcation and would have led to branch vessel occlusion and loss of functional renal parenchyma.
Table 1.
Clinical and operative findings in three patients having open resection of a renal artery aneurysm
| Variable | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age, years | 52 | 65 | 63 |
| Sex | Male | Female | Female |
| Renal artery aneurysm size, cm | 2 | >2 | 1.1 |
| Type of renal artery aneurysm | 1 | 2 | 1 |
| Laterality | Right | Right + left | Left |
| Contraindication to endovascular surgery | Posterior branch off of aneurysm | Tortuosity of proximal vessel | Aneurysm at renal artery bifurcation |
| Hepatic pathology | Child-Pugh Class A cirrhosis | Liver transplant | None |
| Postoperative renal function | Normal | Normal | Normal |
| Hypertension | Negative | Positive | Negative |
| Length of hospital stay, days | 3 | 5 | 3 |
In all cases, a subcostal or transverse abdominal incision was made; the renal veins and the main renal artery were carefully dissected out into the primary branches with careful mobilization of the RAA (Figure 1). After cross-clamping the main renal artery and the distal branches, the aneurysm was opened and the excess wall excised. The branch that originated off of the RAA was spatulated and sewn over the defect in the main renal artery and the other branch to reconstruct the bifurcation. After the anastomosis was completed, flow was restored and confirmed through Doppler ultrasound.
Figure 1.
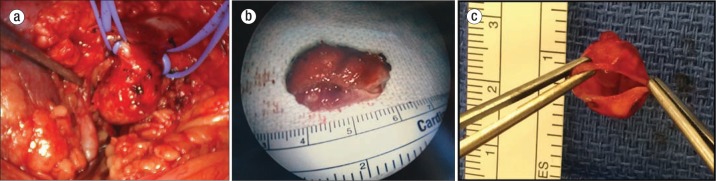
(a) Large aneurysm of renal artery. (b) Excised renal artery aneurysm. (c) Surgical specimen demonstrating the thin-walled aneurysm sac.
DISCUSSION
Historically, autopsy indicated an RAA incidence rate of about 0.03% to 0.09%, but the use of angiography and computed tomography (CT) angiogram (Figure 2) has led to increased detection, which raises the estimated incidence rate to 1% (1, 2). Hypertension is the most common presenting symptom (up to 90%) (1, 2). It has been hypothesized that kinking or twisting of the renal artery causes altered blood flow or embolization, inducing a renin-mediated elevation of blood pressure (5, 6). A more dramatic presentation is seen with rupture of an RAA, which leads to life-threatening hemorrhage associated with a 10% mortality rate (2). Risk factors for rupture include pregnancy, polyarteritis nodosa, and noncalcified and symptomatic RAAs (3). Two of our patients had a history of liver disease, and this is the first time that association has been reported.
Figure 2.
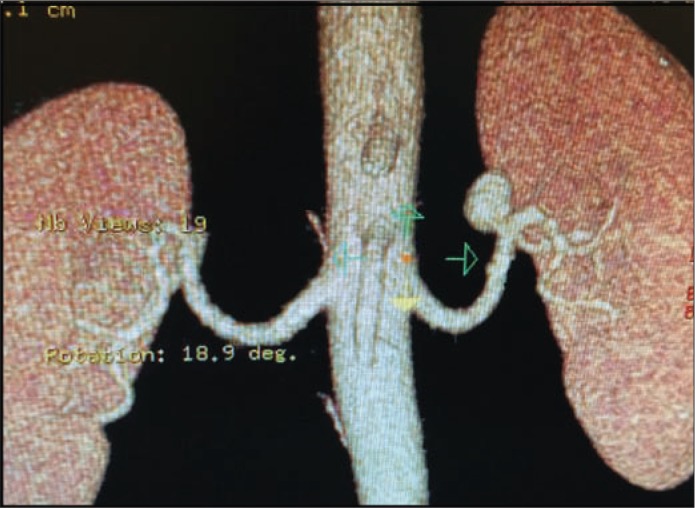
Computed tomography angiogram demonstrating aneurysm at the left renal artery.
Most RAAs are found incidentally on imaging such as CT, angiography, magnetic resonance imaging, and duplex ultrasonography (1). The average RAA is 1.5 cm with a growth rate of approximately 0.6 mm per year (7). RAAs are defined as a dilation of the renal vasculature exceeding twice its normal diameter, and current guidelines suggest surgical repair of RAAs > 2 cm in patients without risk factors. Many RAAs will therefore require regular surveillance (usually with duplex ultrasonography) every 6 to 12 months (1). For one of our patients, the RAA was repaired at a smaller size because of expansion of the patient's saccular aneurysm.
Rundback et al classified RAA into three categories (Figure 3) (2). Saccular aneurysms arising from the main renal artery or the large segmental branch are amendable to an endovascular approach and are classified as type 1. Fusiform aneurysms require an open surgical approach and are considered type 2. Intralobar aneurysms arising from small segmental arteries or accessory arteries are classified as type 3 and can be repaired endovascularly (4).
Figure 3.
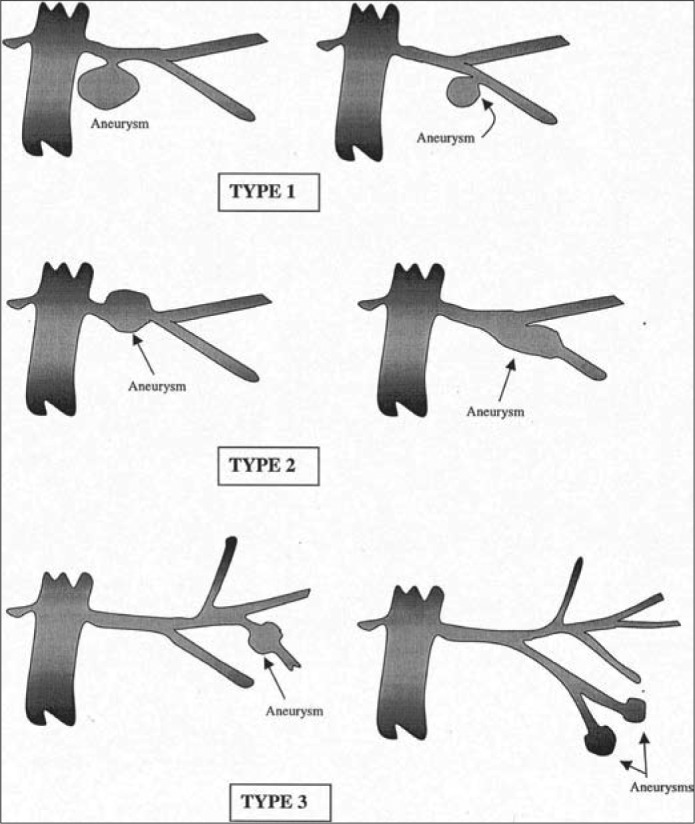
Angiographic classification of renal artery aneurysms. Reprinted from Rundback et al, 2000 (4), with permission from Elsevier.
Traditionally, aneurysmectomy is the most commonly described method to surgically treat RAAs (1). This approach is generally well tolerated, with a 94% long-term aneurysmectomy patency rate at a mean follow-up of 99 months (8). Arteriorrhaphy with or without patch arteriography is a popular approach when dealing with saccular aneurysms, as these present with only a portion of degenerated arterial wall (1). A more novel approach, an ex vivo repair, uses techniques similar to renal transplantation, in which the involved renal vessels are clamped and ligated (1, 3). The kidney is intermittently flushed with cold preservative solution (such as normal saline) and chilled on ice slush. The aneurysm is resected, and the arteries are reanastomosed (1). Nephrectomy is usually an unplanned event necessitated by complications such as aneurysm rupture or end-stage ischemic nephropathy (1).
The placement of covered stents and embolization are two popular approaches to managing RAAs endovascularly. The management of wide-necked RAAs involves stent-assisted coil embolization, which can occlude the aneurysm by deploying a nitinol stent across the neck of the aneurysm. A microcatheter is then introduced into the aneurysmal sac, and detachable coils are packed into the aneurysm (5).
Tsilimparis et al compared endovascular versus open surgical repair and described equivalent perioperative morbidity without mortality and a similar risk for cardiac, respiratory, and renal complications at 30 days (9). Endovascular repair has the advantage of being a noninvasive procedure that can be performed under local anesthesia and with a short hospitalization (10). However, only a subset of patients fit the anatomic criteria to undergo this approach. Limitations include distal torturous arteries, which are not easily accessed by large and rigid delivery systems; small arteries (<6 mm), which can thrombose during covered stent placement (5); and the lack of appropriate proximal and distal landing zones. Complications include nontarget embolization, worsening hypertension, stent thrombus, infection, and radiation skin burns (11). In their literature review, Cochennec et al found that 7.9% of patients with endovascular repair experienced an occurrence of reperfusion of the aneurysm within 10 months to 78 months (8). Open surgery for RAAs shares complications of any major abdominal surgery, with RAA-specific complications of renal artery/graft occlusion, prothrombotic nature of some graft material, segmental ischemia through emboli migration during repair, and diminished renal function secondary to prolonged warm ischemia time (2). However, low perioperative morbidity and mortality have been reported, with only rare occurrences of complications (8). In our case series, all patients continue to do well and have retained normal renal function at 4 months to 7 months postoperatively.
References
- 1.Orion KC, Abularrage CJ. Renal artery aneurysms: movement toward endovascular repair. Semin Vasc Surg. 2013;26(4):226–232. doi: 10.1053/j.semvascsurg.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 2.González J, Esteban M, Andrés G, Linares E, Martínez-Salamanca JI. Renal artery aneurysms. Curr Urol Rep. 2014;15(1):376. doi: 10.1007/s11934-013-0376-z. [DOI] [PubMed] [Google Scholar]
- 3.Ufberg JW, Mcneil B, Swisher L. Ruptured renal artery aneurysm: an uncommon cause of acute abdominal pain. J Emerg Med. 2003;25(1):35–38. doi: 10.1016/s0736-4679(03)00101-x. [DOI] [PubMed] [Google Scholar]
- 4.Rundback JH, Rizvi A, Rozenblit GN, Poplausky M, Maddineni S, Crea G, Agrawal U, Olson C, Matalon TA. Percutaneous stent-graft management of renal artery aneurysms. J Vasc Interv Radiol. 2000;11(9):1189–1193. doi: 10.1016/s1051-0443(07)61362-1. [DOI] [PubMed] [Google Scholar]
- 5.Cura M, Elmerhi F, Bugnogne A, Palacios R, Suri R, Dalsaso T. Renal aneurysms and pseudoaneurysms. Clin Imaging. 2011;35(1):29–41. doi: 10.1016/j.clinimag.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Henke PK, Cardneau JD, Welling TH, 3rd, Upchurch GR, Jr, Wakefield TW, Jacobs LA, Proctor SB, Greenfield LJ, Stanley JC. Renal artery aneurysms: a 35-year clinical experience with 252 aneurysms in 168 patients. Ann Surg. 2001;234(4):454–462. doi: 10.1097/00000658-200110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klausner JQ, Harlander-Locke MP, Plotnik AN, Lehrman E, Derubertis BG, Lawrence PF. Current treatment of renal artery aneurysms may be too aggressive. J Vasc Surg. 2014;59(5):1356–1361. doi: 10.1016/j.jvs.2013.11.062. [DOI] [PubMed] [Google Scholar]
- 8.Robinson WP, Bafford R, Belkin M, Menard MT. Favorable outcomes with in situ techniques for surgical repair of complex renal artery aneurysms. J Vasc Surg. 2011;53(3):684–691. doi: 10.1016/j.jvs.2010.10.050. [DOI] [PubMed] [Google Scholar]
- 9.Tsilimparis N, Reeves JG, Dayama A, Perez SD, Debus ES, Ricotta JJ. Endovascular vs open repair of renal artery aneurysms: outcomes of repair and long-term renal function. J Am Coll Surg. 2013;217(2):263–269. doi: 10.1016/j.jamcollsurg.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Abdalla S, Pierret C, Ba B, Mlynski A, de Kerangal X, Houlgatte A. Surgical treatment of an aneurysm of a distal branch of the renal artery. Ann Vasc Surg. 2014;28(1):260.e9–e12. doi: 10.1016/j.avsg.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Khinda J, Athreya S. Endovascular intervention in renovascular disease: a pictorial review. Insights Imaging. 2014;5(6):667–676. doi: 10.1007/s13244-014-0363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


