Summary
Background
Gynecomastia (GM) is a benign condition with glandular tissue enlargement of the male breast. GM is classified into 4 grades of increasing severity. We describe a series of GM grade I-II, diagnosed, treated surgically and analyzed regarding feasibility, complication rate, and satisfaction.
Methods
From 2005 to 2012, a chart review was performed for 53 patients. Preoperative examination included endocrine and urological examination and exclusion of other pathological conditions. The surgical technique consisted of liposuction through an inframammarian-fold incision and excision of the glandular tissue by a minimal periareolar approach.
Results
A total number of 53 male patients with 104 breasts were available for analysis. By liposuction, a median of 300 ml (range: 10-1000 ml) was aspirated from each breast and 25.1 g (range: 3-233 g) gland tissue was resected. Surgery lasted between 25 and 164 min per patient (median: 72 min). 2 postoperative hemorrhages occurred (n = 2, 3.8%). 2 patients underwent re-operation due to cosmetic reasons (n = 2, 3.8%).
Conclusions
This analysis demonstrates that treatment of GM grade I-II can easily be performed by liposuction combined with subcutaneous resection of the glandular tissue as a minimally invasive and low-impact surgical treatment with a low rate of complications and excellent patient satisfaction. Preoperative workup is important to rule out specific diseases or malignancy causing the GM.
Keywords: Gynecomastia, Liposuction, Subcutaneous mastectomy, Periareolar mastopexy
Introduction
Gynecomastia (GM) is a common condition but with variable severity that occurs in men at all ages and refers to a benign condition of breast enlargement with benign glandular proliferation of various causes. It can be found at autopsy in 40-55% of all men [1,2,3,4,5]. GM most often presents bilaterally, although it can occur asymmetrically. Patients with GM vera most frequently present suffering from aesthetic or psychological symptoms [6].
Three peaks in the prevalence of palpable GM are described: The first one occurs in infants, caused by high materno-placental estrogen levels, and regresses in the first weeks after birth [7]. The second peak appears during puberty between the ages of 13 and 14 years, with a high rate of regression before the 17th year of life [8,9,10]. This transient form is related to the hormonal changes during puberty, which shows an earlier peak of estrogen before testosterone reaches its peak levels. The third peak occurs in men between 50 and 80 years of age related to diverse conditions [4]. Besides physiological categories, GM can be categorized by the triggers (25% persistent pubertal GM, 10-25% drug induced, 8% cirrhosis or malnutrition, 10% male hypogonadism, 3% testicular tumors, 1.5% hyperthyroidism, 1% chronic renal insufficiency, 25% idiopathic) [11]. GM results from an increased volume of glandular tissue or adipose tissue (pseudogynecomastia) or a mixture of both. All causes share an imbalance of androgens and estrogens with a decreased testosterone-to-estradiol ratio, which directly affects the breast tissue [12]. Altered ratios of androgens and estrogens or the increased sensitivity of breast tissue to normal circulating estrogen levels gives rise to ductal hyperplasia, elongation and branching of the ducts correlated with proliferation of fibroblasts and neovascularization [13,14], which altogether form the clinical appearance of GM vera (fig. 1).
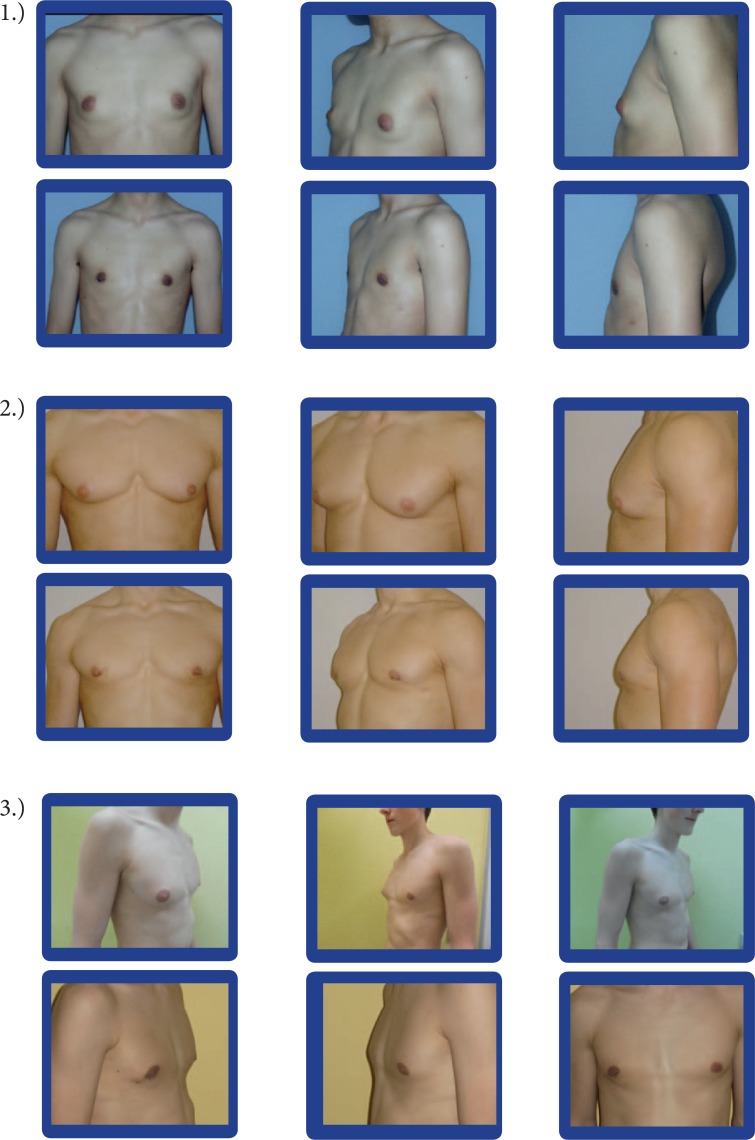
Fig. 1.
All pictures of each section (1-3) show the same patient before (upper row) and 3 months after (lower row) surgical therapy (same room, light, camera, camera settings, and photographer). In all cases, a bilateral s.c. mastectomy and a bilateral liposuction were performed.
To categorize GM clinically, the classification of Simon is most often used [15]. It groups patients into categories according to the size of the GM. Group I is characterized by minor but visible breast enlargement without skin redundancy. Group IIA features moderate breast enlargement without skin redundancy. Group IIB shows moderate breast enlargement with minor skin redundancy. Group III is characterized by gross breast enlargement with skin redundancy that simulates a pendulous female breast. Patients in groups I and II do not require skin excision during potential surgery, but the breast development associated with group III is so marked that excess skin must be removed. Therapy of GM depends on several factors including its duration, grade, etiology, and the presence or absence of clinical symptoms. Treatment options include drug therapy and surgical removal of breast glandular tissue [16,17]. After a persistence of 12 months or longer, GM is likely to have reached the fibrotic status, in which conservative treatment is not effective.
The aim of this study was to analyze the surgical strategies in GM grade I-II, their clinical outcome and, based on this data, the development of an algorithm for surgical treatment.
Patients and Methods
Between 2005 and 2012, we surgically treated 53 patients (a total of 104 breasts) with GM vera grade I-II at the University Hospital of Bonn, Germany. We consecutively performed a chart review regarding the following parameters: personal data, preoperative workup, surgical data, histopathological findings, and follow-up.
All patients underwent presurgical workup containing a physical examination, as well as breast ultrasound and/or mammography. If any abnormalities were seen within the ultrasonography, we initiated additional steps of examination like mammography, breast magnetic resonance imaging (MRI) or fine-needle biopsy.
Low costs, high sensitivity, high specificity, lack of X-rays, and the broad availability make breast ultrasound the first – and, if inconspicuous, only – imaging technique required before surgery. The physical examination consisted of abdominal and urological assessment (including testicular and renal ultrasonography), which is required to exclude differential diagnoses (testicular tumors, malnutrition, underlying liver diseases, hypogonadism, hyperthyroidism, chronical renal insufficiency, and others). Laboratory workup is employed to examine several causes of GM (liver enzymes, creatinine, potassium, sodium, calcium, thyroid-stimulating hormone, free triiodothyronine, luteinizing hormone/follicle-stimulating hormone ratio, testosterone, estradiol, sexual hormone-binding globulins, prolactin, human choriongonadotropin β subunit (β-HCG)). Preoperative markings were done in vertical position after photographic documentation. In this retrospective analysis, we excluded pseudogynecomastia (pseudogynecomastia refers to an enlargement of the male breast by an excess of adipose tissue without any increase in breast tissue) patients for surgery (exclusion was made clinically and sonographically).
Surgical Technique
Subcutaneous fatty tissue of the breast was infiltrated with Standard Tumescent Solution [18]. Standard Tumescent Solution consists of 50 ml prilocaine 1%, 1 ml epinephrine (1:1,000), 8 ml sodium bicarbonate 8.4%, 1 ml triamcinolone acetonide, and 1,000 ml physiological saline [19]. 2 stab incisions per side were made by scalpel in the inframammary fold, 1 at the medial and 1 at the lateral aspect of the inframammary fold. Depending on the thickness of the adipose tissue layer, liposuction of the deeper layers was performed with a 4-mm cannula, followed by superficial or fine-contouring liposuction using a 3-mm cannula. During the liposuction, the gland was partially dissected from the pectoral fascia and the surrounding tissue. After liposuction, the residual gland was removed from the retroareolar area via periareolar incision (in the median third of the areolar circumference), while pulling the nipple-areolar complex ventrally with a double-hook retractor. Preparation was then performed in direction of the thoracic wall, excising the glandular tissue under visual control. Only a thin retroareolar glandular tissue layer was left in place in order to guarantee substantial blood supply to the areolar complex. If necessary, final or additional contour-shaping liposuction was easily performed through the incision under direct visualization. To minimize the wound cavity and the wound surface, 3-0 absorbable adaptive and subcuticular situative sutures were placed, followed by a running intradermal 4-0 absorbable suture. Sterile dressing was applied and moderate pressure was administrated to the thoracic wall by a non-wire, front-closure compression vest for approximately 4 weeks, which reduces hematoma and seroma formation to improve the aesthetic outcome. Regular follow-up visits were performed on the first postoperative day and 2 weeks and 6 months after surgery.
Results
Clinical data from 53 patients and 104 male breasts were available for analysis. Patients were treated unilaterally in 2 cases (n = 2, 3.8%) and bilaterally in 51 cases (96.2%), from 2005 to 2012. The mean age was 28.2 years (range: 13-66 years). The only adolescent was 13 years of age, suffering from bilateral GM grade II with severe pain and tenderness, without spontaneous regression for 11 months. The mean weight was 82.6 kg (range: 60.1-116 kg) and the mean body mass index (BMI) was 25.3 (range: 20.5-36.2). Most of the patients underwent a bilateral subcutaneous (s.c.) mastectomy plus bilateral liposuction (n = 44, 83%). 4 patients had only bilateral liposuction (7.5%) and 3 patients had only a bilateral s.c. mastectomy (5.7%), whereas the 2 patients with unilateral GM vera were both treated with unilateral s.c. mastectomy plus unilateral liposuction (3.8%). On average, 300 ml (range: 10-1,000 ml) was aspirated and 25.1 g (range: 3-233 g) gland tissue was resected from each breast.
Surgery lasted between 25 (unilateral surgery) and 164 min per patient (median 72 min; time from incision to suture). In all cases, the pathological examination revealed GM vera. The only postoperative complication observed was postoperative hemorrhage in 2 cases (3.8%). There were no hematomas, seromas, infections, or non-resolving hypesthesias observed during follow-up. 2 patients underwent re-operation due to relapse of the GM after 12 and 15 months (n = 2, 3.8%). Both patients who relapsed were primarily treated with liposuction alone.
During the follow-up course, all patients expressed satisfaction with the aesthetic results; no pain was asserted. The post-operative residence time in hospital varied from 1 to 3 days (on average, 1.2 days).
Discussion
Surgery is by far the main strategy and the most efficient way in the therapy of GM grade I-II in adults. Our results suggest that s.c. mastectomy combined with liposuction should be performed as the preferable operation technique, justified by low complication and recurrence rates and excellent aesthetical outcomes. Using the liposuction technique as the first step leads to the formation of an optically appealing breast and simplifies the following excision steps by defining the glandular tissue [20]. The typical retroareolar rubber most often cannot be removed by the liposuction cannulas alone without complications [21,22,23], even with special 2.3-mm cannulas [24,25], sharpened cannulas [26], or other efforts [27] made so far. In our evaluation, the only patients suffering from relapse of GM were those primarily treated by liposuction alone. Therefore, we strongly recommend excising the retroareolar glandular tissue in patients suffering from GM. Omitting the retroareolar gland resection was described as the major complication in trials evaluating liposuction alone [28]. On the other hand, by performing the liposuction as the first step of surgery, the retroareolar gland is demarcated from the surrounding tissue and is therefore easier to resect. Thus, the combination of both methods – liposuction followed by glandular removal – guarantees excellent aesthetic results.
With a post-operative residence time in hospital of 1.2 days on average, costs are low. They are economically lower than those for conservative treatment options or psychological support and, perhaps, for a following surgical approach due to therapy failure in a conservative approach. Liposuction combined with s.c. mastectomy is a very effective treatment with high satisfaction rates together with few complications during the entire course of therapy. By combined surgery, aesthetic satisfaction and relief of pain and tenderness can be rapidly achieved, with a lower rate of recurrences. An accurate presurgical workup is the basis of successful surgery with excellent aesthetic results. Preoperative sonography is strictly recommended to plan the extent of liposuction due to the estimated mastectomy volume. Low costs, high sensitivity, high specificity, lack of X-rays, and the broad availability make breast ultrasound the only imaging technique required before surgery. Only in the case of pathologic findings during the breast ultrasound, other imaging techniques like mammography and, if necessary, fine-needle core biopsy are required. The indication for an adapting liposuction is almost always given, even in thin patients. It is worth discussing whether surgery should be indicated even in obese patients just to give them a positive psychological signal since, even according to the general references, weight loss should be the first step in these patients. Observation for 6-12 months is reasonable in these cases (in the absence of suspected malignancy). Patients with GM caused by drugs should be evaluated for the necessity of taking these drugs or for switching to alternative drugs treating the underlying disease. Conservative trials may be effective in the early, florid phase of GM. 3 types of drugs have been evaluated but not approved by the Food and Drug Administration (FDA) in small cohorts: aromatase inhibitors (AIs), selective estrogen receptor modulators (SERMS), and androgens [17,29]. Complete regression has rarely been reported (at small percentages); partial regression and especially relief of pain and tenderness have often been reported. Medical treatment for this disease is long lasting; the success rates are low, whereas surgical treatment is mostly straightforward. Patients suffering from pseudogynecomastia may relapse, even if the glandular tissue was excised in toto.
The difficulties of reimbursement of surgery by health insurances and benefactors are nearly impregnable, although the patients frequently present with a high psychological strain. However, these patients could be treated efficiently and cost effectively [30].
Nowadays, the elevated body awareness will raise the discussion of whether surgical therapy of GM should be used more often in the future. Therefore, the indication for surgical therapy of GM should be made more generously and more frequently due to the feasibility shown in this study.
Disclosure Statement
The authors have no disclosures to make relevant to this manuscript.
References
- 1.Braunstein GD. Clinical practice. Gynecomastia. N Engl J Med. 2007;357:1229–1237. doi: 10.1056/NEJMcp070677. [DOI] [PubMed] [Google Scholar]
- 2.Rahmani S, Turton P, Shaaban A, Dall B. Overview of gynecomastia in the modern era and the Leeds Gynaecomastia Investigation algorithm. Breast J. 2011;17:246–255. doi: 10.1111/j.1524-4741.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- 3.Ley SJ. Cardiac surgery in an era of antiplatelet therapies: generating new evidence. Reflect Nurs Leadersh. 2002;28:35. [PubMed] [Google Scholar]
- 4.Georgiadis E, Papandreou L, Evangelopoulou C, et al. Incidence of gynaecomastia in 954 young males and its relationship to somatometric parameters. Ann Hum Biol. 1994;21:579–587. doi: 10.1080/03014469400003582. [DOI] [PubMed] [Google Scholar]
- 5.Nuttall FQ. Gynecomastia as a physical finding in normal men. J Clin Endocrinol Metab. 1979;48:338–340. doi: 10.1210/jcem-48-2-338. [DOI] [PubMed] [Google Scholar]
- 6.Rosen H, Webb ML, DiVasta AD, Greene AK, Weldon CB, Kozakewich H, Perez-Atayde AR, Labow BI. Adolescent gynecomastia: not only an obesity issue. Ann Plast Surg. 2010;64:688–690. doi: 10.1097/SAP.0b013e3181dba827. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Voigt J. Brustdrüsenschwellungen bei männlichen Jugendlichen des Pubertätsalters (Pubertätsmakromastie) Z Kinderheilkd. 1941;62:590–606. [Google Scholar]
- 8.Moore DC, Schlaepfer LV, Paunier L, Sizonenko PC. Hormonal changes during puberty: V. Transient pubertal gynecomastia: abnormal androgen-estrogen ratios. J Clin Endocrinol Metab. 1984;58:492–499. doi: 10.1210/jcem-58-3-492. [DOI] [PubMed] [Google Scholar]
- 9.Harlan WR, Grillo GP, Cornoni-Huntley J, Leaverton PE. Secondary sex characteristics of boys 12 to 17 years of age: the U.S. Health Examination Survey. J Pediatr. 1979;95:293–297. doi: 10.1016/s0022-3476(79)80677-0. [DOI] [PubMed] [Google Scholar]
- 10.Lee PA. The relationship of concentration of serum hormones to pubertal gynecomastia. J Pediatr. 1995;86:212–215. doi: 10.1016/s0022-3476(75)80470-7. [DOI] [PubMed] [Google Scholar]
- 11.Braunstein GD. Gynecomastia. N Engl J Med. 1993;328:490–495. doi: 10.1056/NEJM199302183280708. [DOI] [PubMed] [Google Scholar]
- 12.Mathur R, Braunstein GD. Gynecomastia: pathomechanisms and treatment strategies. Horm Res. 1997;48:95–102. doi: 10.1159/000185497. [DOI] [PubMed] [Google Scholar]
- 13.Nicolis GL, Modlinger RS, Gabrilove JL. A study of the histopathology of human gynecomastia. J Clin Endocrinol Metab. 1971;32:173–178. doi: 10.1210/jcem-32-2-173. [DOI] [PubMed] [Google Scholar]
- 14.Bannayan GA, Hajdu SI. Gynecomastia: clinicopathologic study of 351 cases. Am J Clin Pathol. 1972;57:431–437. doi: 10.1093/ajcp/57.4.431. [DOI] [PubMed] [Google Scholar]
- 15.Simon BE, Hoffman S, Kahn S. Classification and surgical correction of gynecomastia. Plast Reconstr Surg. 1973;51:48–52. doi: 10.1097/00006534-197301000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Gikas P, Mokbel K. Management of gynaecomastia: an update. Int J Clin Pract. 2007;61:1209–1215. doi: 10.1111/j.1742-1241.2006.01095.x. [DOI] [PubMed] [Google Scholar]
- 17.Gruntmanis U, Braunstein GD. Treatment of gynecomastia. Curr Opin Investig Drugs. 2001;2:643–649. [PubMed] [Google Scholar]
- 18.Venkataram J, Venkataram M. Liposuction and the cutaneous surgeon. J Cutan Aesthet Surg. 2013;6:129–131. doi: 10.4103/0974-2077.118401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248–263. doi: 10.1111/j.1524-4725.1990.tb03961.x. [DOI] [PubMed] [Google Scholar]
- 20.Samdal F, Kleppe G, Amland PF, Abyholm F. Surgical treatment of gynaecomastia. Scand J Plast Reconstr Hand Surg. 1994;28:123–130. doi: 10.3109/02844319409071189. [DOI] [PubMed] [Google Scholar]
- 21.Gasperoni C, Salgarello M, Gasperoni P. Technical refinements in the surgical treatment of gynaecomastia. Ann Plast Surg. 2000;44:455–458. doi: 10.1097/00000637-200044040-00020. [DOI] [PubMed] [Google Scholar]
- 22.Courtiss EH. Gynecomastia: analysis of 159 patients and current recommendations for treatment. Plast Reconstr Surg. 1987;79:740–750. doi: 10.1097/00006534-198705000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Lewis CM. Lipoplasty: treatment for gynaecomastia. Aesthetic Plast Surg. 1985;9:287–292. doi: 10.1007/BF01571048. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg GJ. Gynecomastia: suction lipectomy as a contemporary solution. Plast Reconstr Surg. 1987;80:379–385. [PubMed] [Google Scholar]
- 25.Rosenberg GJ. A new cannula for suction removal of parenchymal tissue of gynaecomastia. Plast Reconstr Surg. 1994;94:548–551. doi: 10.1097/00006534-199409000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Samdal F, Kleppe G, Abyholm F. A new suction-assisted device for removing glandular gynaecomastia. Plast Reconstr Surg. 1991;87:383–384. [PubMed] [Google Scholar]
- 27.Luckey RC. Modified technique for correction of gynecomastia. Plast Reconstr Surg. 1992;89:767. doi: 10.1097/00006534-199204000-00056. [DOI] [PubMed] [Google Scholar]
- 28.Fruhstorfer BH, Malata CM. A systematic approach to the surgical treatment of gynaecomastia. Br J Plast Surg. 2003;56:237–246. doi: 10.1016/s0007-1226(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 29.Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab. 1999;84:3469–3478. doi: 10.1210/jcem.84.10.6078. [DOI] [PubMed] [Google Scholar]
- 30.Wolter A, Scholz T, Diedrichson J, Liebau J. Surgical treatment of gynecomastia: an algorithm. Handchir Mikrochir Plast Chir. 2013;45:73–79. doi: 10.1055/s-0033-1334910. [DOI] [PubMed] [Google Scholar]