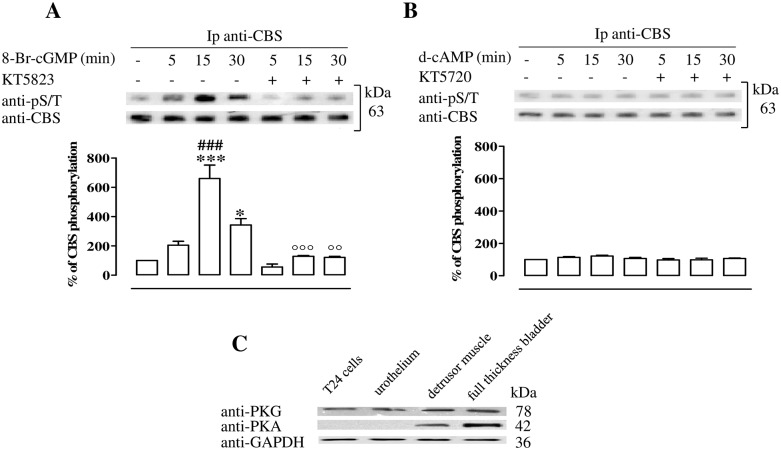
Fig 4. PKG phosphorylates CBS in T24 cells.
(A) CBS is isolated by immunoprecipitation (Ip) from starved T24 cells treated with 8-Br-cGMP or vehicle in the presence or the absence of PKG inhibitor. CBS phosphorylation is evaluated by western blotting assay using anti-pS/T. 8-Br-cGMP treatment causes a time-dependent increase of CBS phosphorylation (*p<0.05, ***p<0.001 vs vehicle; ### p<0.001 vs 5 and 30 min.). PKG inhibition (KT5823) abolishes CBS phosphorylation induced by the 8-Br-cGMP (°°p<0.01 and °°°p<0.001 vs matched time point). (B) CBS is isolated by Ip from starved T24 cells treated with d-cAMP or vehicle in the presence or absence of PKA inhibitor. CBS phosphorylation is evaluated by western blotting assay using anti-pS/T. The stimulation with d-cAMP in the presence or in the absence of PKA inhibitor, KT5720, does not cause changes in phosphorylated CBS level. Data are calculated as % of CBS phosphorylation and expressed as mean ± SE of three separate experiments. (C) Representative protein expression of PKG and PKA in human T24 urothelial cells, urothelium, detrusor muscle and full thickness bladder tissue homogenates. Loading in the gel lanes was controlled by detection of GAPDH protein. PKG is expressed in all samples. PKA is highly expressed in human detrusor and whole bladder but not in T24 cell lines or human urothelium. The blot is representative of three separate experiments.