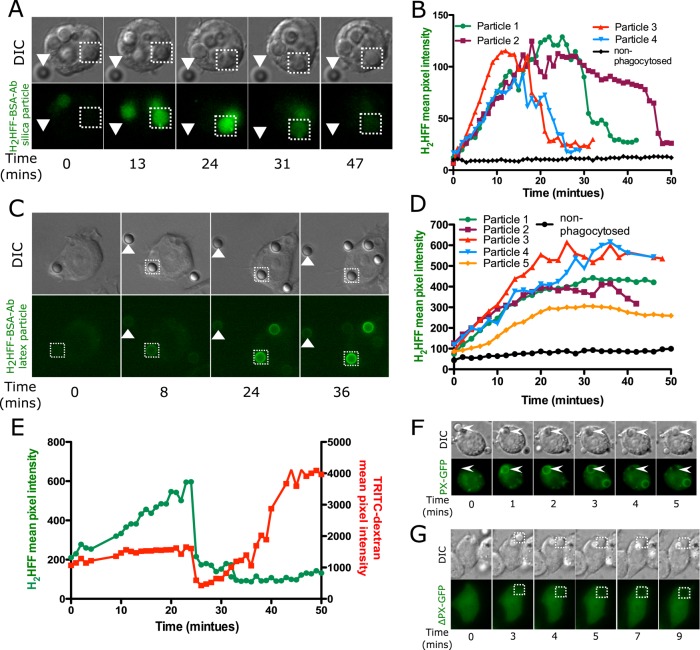
FIGURE 4:
Phagosomal ROS is detected upon uptake of silica or latex particles. H2HFF-BSA was coupled to particles, and then the particles were imaged after uptake into MH-S macrophage cells using a wide-field epifluorescence microscope. (A) Phagosomes containing opsonized silica particles labeled with H2HFF-BSA (square) show an increase in fluorescence indicative of ROS generation. The fluorescence of the phagosome later decreased, indicative of leakage. Nonphagocytosed particles (triangle) did not show any change in fluorescence. (B) Quantification of fluorescence of several particles from several cells. Particle 1 is the particle indicated by the square in A. (C) Latex particles labeled with H2HFF-BSA also showed an increase in fluorescence (square) indicative of ROS generation. Nonphagocytosed particles (triangle) do not show an increase in fluorescence (control). (D) Quantification of the fluorescence from C and other cells. Particle 1 represents quantification of particle indicated by the square in C. (E) To confirm a decrease in H2HFF fluorescence observed in A is due to phagolysosomal leakage, macrophages loaded with TRTIC-dextran were exposed to H2HFF-BSA–labeled silica particles. A decrease in TRITC-dextran and H2HFF fluorescence was observed simultaneously at 25 min, confirming that the decrease is due to phagolysosomal leakage. (F) MH-S cells were transiently transfected with a vector expressing the PX domain of p40phox fused to GFP (PX-GFP). On uptake of opsonized silica particles, PX-GFP localized around the phagosomes. (G) Cells were transiently transfected with a vector expressing the PX domain, where arginine at position 42 is replaced by glutamine, fused to GFP (ΔPX-GFP), and expressed in macrophages. This probe does not show localization around phagosomes.