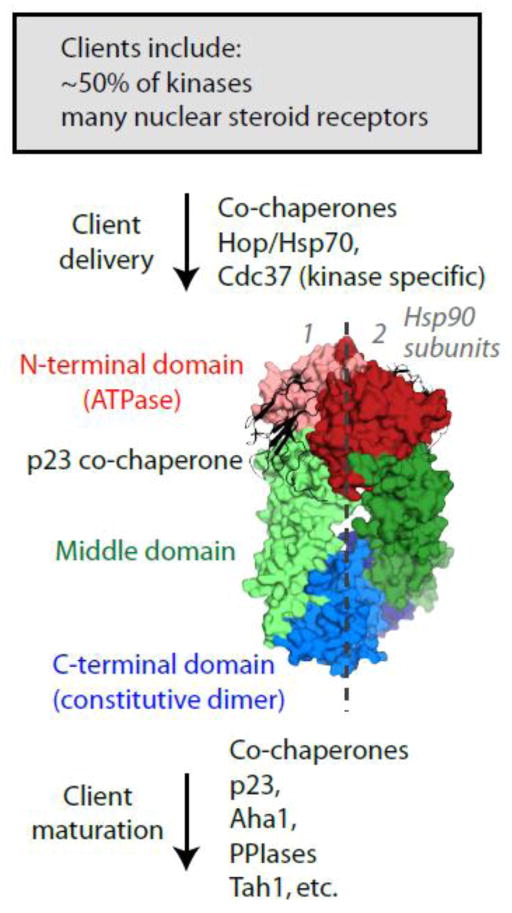
Figure 1.
Hsp90 functions together with multiple co-chaperones to mature many signal transduction clients to their active states. The middle panel shows a molecular illustration based on a symmetric crystal structure (2CG9.PDB36) of the Hsp90 homodimer shown in surface representation bound to two monomers of the p23 co-chaperone shown in ribbon form. While many aspects of Hsp90 mechanism remain unclear, recent structural and biochemical studies have demonstrated that each subunit in the Hsp90 dimer interacts asymmetrically with clients during the maturation process.