Figure 3.
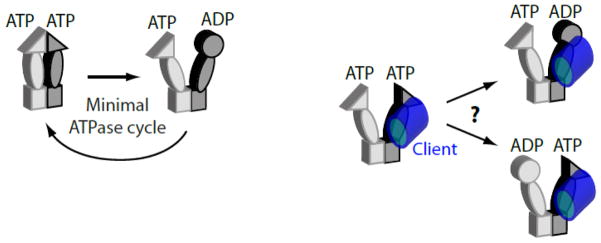
Investigating the function of each Hsp90 subunit in vivo. Analysis of engineered Hsp90 heterodimers in yeast indicate that ATP binding is required in both subunits, but that ATP hydrolysis is only critical in one subunit (left panel). Engineered Hsp90 heterodimers may enable future studies to investigate the asymmetric operating principles of this chaperone including if client maturation depends on which subunit hydrolyzes ATP relative to which subunit forms the primary binding contact to client (right panel).
