Abstract
Objective
To identify survival differences in patients with sarcomatoid prostate cancer based on initial staging and treatment regimens.
Methods
We retrospectively reviewed the clinical outcomes of patients with a pathologically confirmed diagnosis of sarcomatoid prostate cancer. The primary statistical objective was to estimate overall survival and assess the survival of patients at different stages treated with either local and/or systemic approaches.
Results
We identified 70 transurethral resections, needle biopsies or prostatectomy specimens that were reviewed by the Department of Pathology at Johns Hopkins Hospital from 2002–2012 and given the diagnosis of sarcomatoid prostate cancer. Of the 45 patients with available survival data, complete medical histories were obtained on 27 patients who were stratified based on a modified staging system (local disease, local disease with bladder invasion, and metastatic disease). After a median follow-up of 106 months, the median overall survival (OS) of patients in the local disease group was not reached. Notably, five of the 9 patients diagnosed with local disease survived > 5 years and were treated with surgery and/or external beam radiation therapy. The OS hazard was significantly increased in patients with either clinical evidence of bladder invasion (HR: 20.46 [95% CI: 2.43,172]; p = < 0.0001) or metastatic disease (HR: 43.34 [95% CI: 4.39,427.4]; p = < 0.0001), which both demonstrated poor outcomes (median OS: local with bladder invasion – 9 months; metastatic disease – 7.1 months).
Conclusions
This retrospective analysis suggests that local sarcomatoid prostate cancer can be effectively treated with definitive therapy leading to favorable outcomes.
Keywords: Sarcomatoid, prostate cancer
INTRODUCTION
Sarcomatoid carcinoma is a rare type of prostate cancer representing <1% of all prostate neoplasms. Patients with the disease have a poor prognosis for extended survival.1 These tumors are histologically characterized by a malignant epithelial component and a distinct population of sarcomatoid or mesenchymal-appearing cells.2 Recently, a prostate-specific, ETS-related (erythroblast transformation-specific) gene (ERG) deletion was detected in both the sarcomatoid component and adjacent adenocarcinoma, confirming that these tumors are derived from prostate epithelium.3 Sarcomatoid prostate cancer can develop in the absence of PSA elevation, making it difficult to detect disease progression.4 Several researchers have suggested that prior radiation therapy may predispose the prostate to the development of a sarcomatoid malignancy, but a clear association has not been shown.5–8 Many of the published cases of sarcomatoid prostate cancer have been associated with a prior history of prostate adenocarcinoma. The largest case series published to date consisted of 42 patients, with 66% having had a prior diagnosis of acinar adenocarcinoma.9
Case reports and series available in the literature uniformly demonstrate dismal outcomes.9–15 However, survival outcomes based on stage and response to conventional treatment have not been reported. We retrospectively reviewed the clinical outcomes of patients with a pathologically confirmed diagnosis of sarcomatoid prostate cancer in an effort to identify potential benefits from treatment and better inform prognosis.
METHODS
Patients with sarcomatoid prostate cancer were identified using a Johns Hopkins Hospital Department of Pathology database. Seventy cases of sarcomatoid prostate cancer were reviewed by the Department of Pathology at Johns Hopkins Hospital from 2002–2012. Five of the 70 patients were diagnosed and treated at Johns Hopkins Hospital. The remaining 65 patients were seen as pathology consults to one of the authors (JE). Patients were treated at the discretion of their primary urologist, medical oncologist, and/or radiation oncologist. The Institutional Review Board (IRB) granted a waiver of consent to contact providers directly regarding each patient’s treatment. For patients treated at Johns Hopkins Hospital (5/70), patient data was obtained from the Johns Hopkins Hospital electronic medical record. The remaining patients (65/70) were treated by outside providers. These providers were contacted via telephone and verbally consented for participation. A standardized script was read by one of the authors (MM) seeking information regarding clinical stage, treatment regimens, time-to-progression (PSA, radiographic), and date of death, when applicable. Providers verbally completed the patient questionnaire and were re-contacted when needed for clarification. If the provider could not be reached or did not wish to provide details about the patient, only the clinical information contained in the Department of Pathology database was included.
Clinical staging included an MRI pelvis or CT chest, abdomen and pelvis in all patients. Bone scans were obtained in 10 of 27 patients with available staging information. PET-CT imaging was also obtained in two patients (1- metastatic, 1 – local), which did not change staging based on prior CT studies. The study investigators did not independently review radiographic imaging performed by providers outside Johns Hopkins Hospital.
Local disease was defined as prostate-confined cancer with or without extracapsular extension, in the absence of radiographic evidence of metastatic disease and bladder invasion. Malignant invasion into the bladder was determined by radiographic imaging and/or direct visualization during cystoscopy. Patients with direct invasion of the tumor into the bladder with no distant metastases were characterized as having local disease with bladder invasion. Patients with metastatic disease had at least one focus of visceral and/or bone metastases on imaging. Biopsy-proven metastatic disease was not required for inclusion in this group.
The primary statistical objective was to estimate overall survival (OS) and assess the survival of patients at different stages treated with either local and/or systemic approaches. OS was estimated using the Kaplan-Meier method, and comparisons were made using the log-rank statistic (Mantel) or the Cox proportional hazards regression model.
RESULTS
A total of 70 patients with sarcomatoid prostate cancer were included in this analysis. Twenty-five patients were excluded from the survival analysis due to lack of available clinical information. Survival data was obtained on 45 patients, of whom 27 had confirmed clinical staging and treatment data. Table 1 lists the clinical and histopathological characteristics of all patients. At the time of diagnosis of sarcomatoid carcinoma, there was co-existing adenocarcinoma in 79% (55/70) of cases. All cases of adenocarcinoma had a Gleason score of 7 or greater. Of the patients with available medical histories, 35 of 45 (78%) had a prior history of adenocarcinoma. The time from diagnosis of adenocarcinoma to sarcomatoid prostate cancer varied, ranging from 9 months to 20 years. Twenty-two tissue specimens were previously stained for PSA. Twenty of 22 patients were reported as PSA negative, with the remaining two patients having focally positive staining. Androgen receptor (AR) expression was not assessed.
TABLE 1.
Summary of Clinical and Histopathologic Characteristics
| Total number of cases | 70 |
| Age in years, average (range) | 70.8 (49 – 88) |
| Clinical follow-up available, n (%) | 45/70 (64%) |
| Prior history of acinar adenocarcinoma (%) | 35/45 (78%) |
| Gleason ≤ 6 | 11/21 (52%) |
| Gleason 7–10 | 10/21 (48%) |
| Time between original diagnosis and sarcomatoid carcinoma in years, average (range) | 8.3 years (9 months – 20 years) |
| Concurrent acinar adenocarcinoma, n (%) | 55 /70 (79%) |
| Gleason 7–8 | 13/36 (36%) |
| Gleason 9–10 | 23/36 (74%) |
| PSA immunostaining | |
| Positive (%) | 0/22 (0%) |
| Negative (%) | 20/22 (91%) |
| Focally positive (%) | 2/22 (9%) |
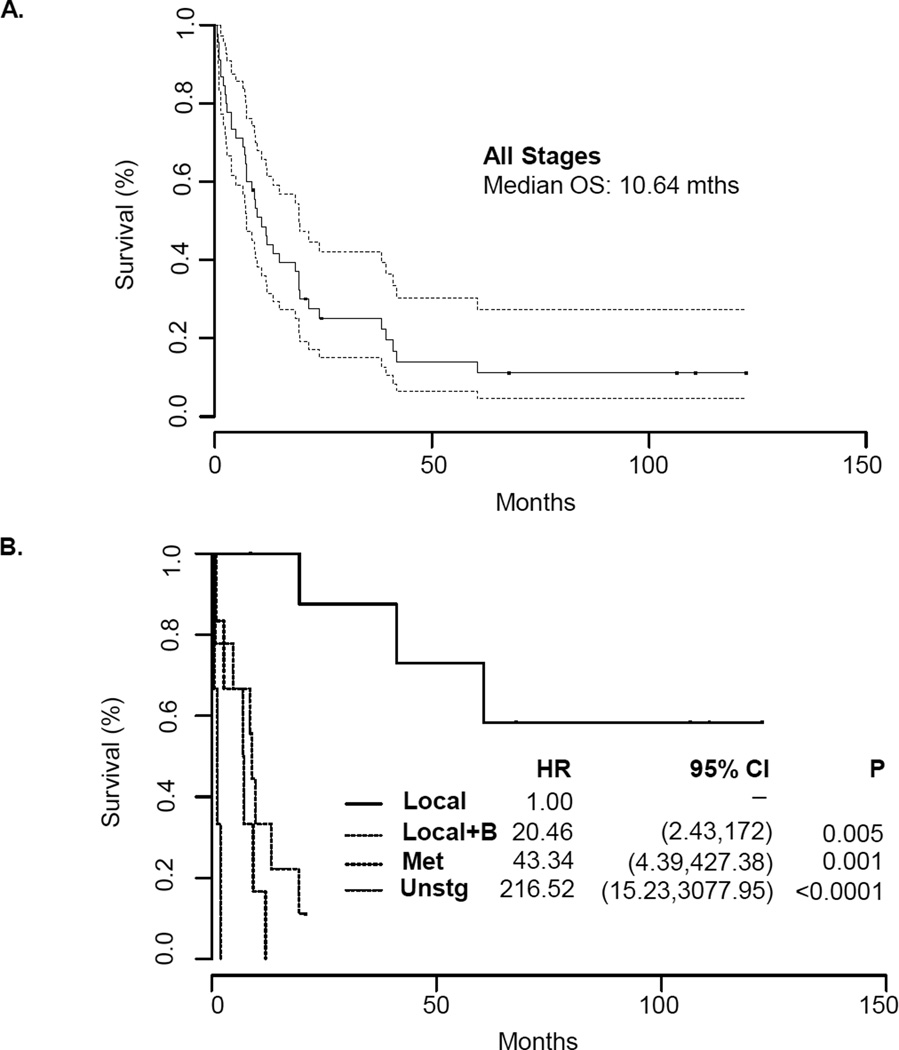
The median OS for the 45 patients with obtainable clinical data was 10.6 months (95% CI: 7.16, 19.38) after a median follow-up of 106 months (Figure 1A). Although the survival for the group as whole was short, outcomes differed substantially according to the extent of the disease. To further define survival outcomes, we subdivided patients based on a modified staging system: local disease, local disease with bladder invasion, metastatic disease, and unstaged disease. After a median follow-up of 106 months, the median OS was not reached in the local disease group. Notably, five of the 9 patients diagnosed with local disease survived > 5 years. For patients with local disease plus bladder invasion and metastatic disease, there was a greater risk of rapid death relative to patients with local disease only (Figure 1B). The OS hazard ratio in the local disease with bladder invasion (median OS: 9 months) and metastatic disease groups (median OS: 7.1 months) were 20.46 (95% CI: 2.43,172; p=0.005) and 43.34 (95% CI: 4.39,427.4; p=0.001), respectively. Unstaged patients were of advanced age (83, 79, and 88 years old) and died shortly after diagnosis, without treatment.
FIGURE 1. Overall Survival of Patients with Sarcomatoid Prostate Cancer.
A. Kaplan Meier estimate of overall survival in all patients with sarcomatoid prostate cancer. The median overall survival was 10.64 months with a subset of patients having prolonged survival. B. Kaplan Meier estimate of overall survival in sarcomatoid prostate cancer patients stratified by a modified staging system. Median OS – local disease: not reached; local with bladder invasion: 9 months; metastatic disease: 7.1 months; unstaged: 1.28 months.
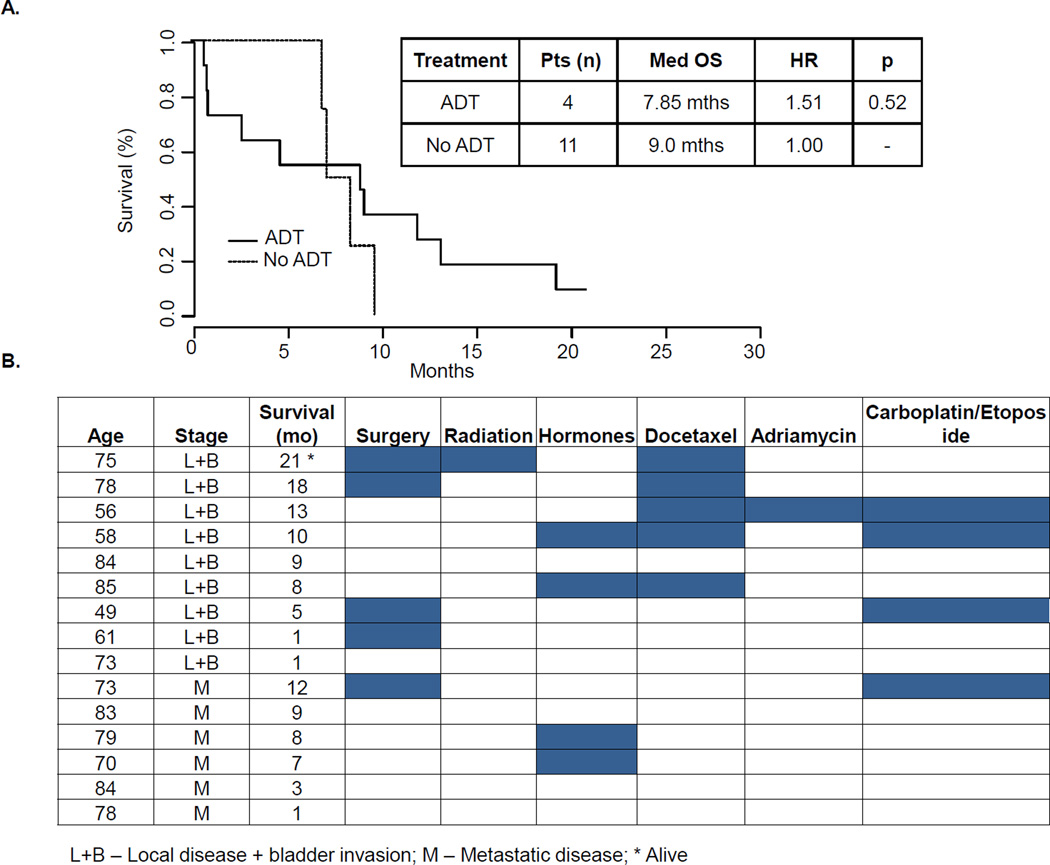
We sought to investigate the outcomes of patients with local disease based on their treatment regimen (Table 2). The majority of patients were alive at the time of analysis (6/9) or had survived more than two years after diagnosis (7/9). Of the six patients alive at the time of analysis, three were treated with surgery alone, two had surgery followed by adjuvant radiation, and one received radiation alone. All patients receiving radiation were treated with external beam radiation. No brachytherapy was administered. Three patients with local disease at diagnosis died due to disease progression, surviving < 7 months after metastatic recurrence.
TABLE 2.
Treatment Paradigms and Outcomes of Patients with Local Disease
| Age | Alive | Survival (mo) | Surgery | Radiation | Hormones |
|---|---|---|---|---|---|
| 61 | Y | 122 | |||
| 51 | Y | 110 | |||
| 56 | Y | 107 | |||
| 79 | Y | 67 | |||
| 66 | N | 60 | |||
| 70 | N | 41 | |||
| 71 | Y | 24 | |||
| 74 | N | 19 | |||
| 79 | Y | 9 |
For further analysis, we combined the patients having metastatic disease with those with local disease with bladder invasion given the comparable poor prognosis of each group. Only four of the 15 patients with advanced disease were treated with hormonal therapy. After a median follow-up of 21 months in the advanced disease group, the four patients receiving gonadotropin-releasing hormone agonists and/or anti-androgens survived 7, 8, 8, and 10 months, while those treated without hormonal therapy survived from 1 to 21 months, with a median of 9 months (Figure 2A). In each of the four patients who received ADT, no PSA or radiographic responses were observed and therapy was stopped within three months. Second-generation anti-androgens (i.e., enzalutamide) and Cytochrome P450 isoform 17 inhibitors (i.e., abiraterone acetate) were not administered.
FIGURE 2. Treatment Paradigms and Outcomes of Patients with Advanced Disease.
A. Fifteen patients were identified with advanced disease. Four of the 15 patients were treated with androgen deprivation therapy with a median survival of 7.85 months (HR 1.51, p = 0.52). Non-hormonal based therapies, used in 11 of 15 patients, led to a median survival of 9 months. B. Patients with advanced disease were grouped based on stage (local disease with bladder invasion and metastatic disease). Treatment paradigms including various chemotherapy regimens are indicated in each column.
Six patients were found to have metastatic disease at the time of diagnosis. The sites of metastatic disease included brain and lung (1/6), bone only (2/6), lung only (2/6), and retroperitoneal lymphadenopathy (1/6). Several different chemotherapy regimens were used to treat patients with advanced disease (Figure 2B). Traditional prostate cancer (single-agent docetaxel) and small cell carcinoma (carboplatin and etoposide) regimens were used in most instances. Of the five patients who received weekly docetaxel, two had no response to treatment and two had partial radiographic and PSA responses lasting four and eight months. One patient had recently started docetaxel at the time of analysis. Four patients were treated with a small cell carcinoma regimen (carboplatin and etoposide). Two of these patients had no radiographic or PSA response after two months of chemotherapy. Partial responses were reported in the remaining two patients during the six cycles of therapy and progressed after cessation of the treatment. One patient was treated with a sarcoma regimen consisting of single-agent adriamycin with rapid disease progression after two cycles. Several patients with advanced disease were not treated, likely due to the overall disease burden, comorbidities, and performance status, and died within nine months after diagnosis.
DISCUSSION
Case reports of sarcomatoid prostate cancer in the literature, prior to our analysis, demonstrate a dismal prognosis.9–15 Given the limited number of cases available at each institution, a more detailed analysis of survival based on stage and treatment has not been performed until now. Patients with local disease at the time of diagnosis did remarkably well. In this group, the median OS was not yet reached after more than eight years of median follow-up. In our analysis, patients in the local disease group were effectively treated with a combination of surgery and/or radiation. Three patients in the local disease group who survived longer than eight years (107, 110, and 122 months) from diagnosis underwent prostatectomy with negative margins on pathology and no lymph node involvement. One of the three patients received adjuvant external beam radiation. While preliminary, this data suggest that patients with clinically localized disease treated with either surgery or radiation or both may have durable remissions and are potentially cured of their disease.
Once invasion into the bladder was noted, OS was similar to the group with metastatic disease, suggesting a lack of an effective systemic therapy. Three patients with bladder invasion underwent cystoprostatectomy as upfront therapy. Two of the three patients died within 5 and 18 months of surgery from disease progression. One patient remained alive at the time of analysis (21 months) and was treated with pelvic exenteration for a local disease recurrence, but subsequently developed distant disease and started on chemotherapy. Although this represents a small number of patients, the poor outcomes of patients with local invasion into the bladder may highlight the propensity of these sarcomatoid tumors to spread systemically, limiting curative options.
This study does not address patients with local disease with lymph node involvement who may be surgical candidates. In our study, one patient in the local disease category was noted to have a single lymph node involved with sarcomatoid carcinoma on surgical biopsy following prostatectomy. At the time of this study, the patient was 24 months out from his initial surgery with no evidence of disease at this time on subsequent imaging. Additional patients would be needed to better characterize the outcomes of local disease with and without lymph node involvement. However, given the dismal outcomes of patients treated with systemic therapies, providers may consider aggressive local therapy (i.e. surgery and/or radiation) in the setting of radiographic pelvic lymph node involvement.
A major limitation of this study is the retrospective design and reliance on the accounts of outside providers to provide a detailed medical history. Although 70 patients were initially identified, many were excluded from the staging and treatment analysis due to lack of reliable clinical information. In many of these instances, medical records were destroyed or incomplete. Moreover, radiology studies were not independently reviewed at a single institution (i.e. Johns Hopkins Hospital) and may have affected staging. In this situation, local disease patients, at worst, would be understaged which would negatively effect the reported survival. However, the survival advantage in this group remained statistically significant.
The favorable outcomes in the local disease group highlight the importance of early diagnosis. In sarcomatoid prostate cancer, the lack of PSA expression by the sarcomatoid component may make it difficult to diagnosis localized disease.4 Of the 22 patients who had PSA staining done at the time of original diagnosis, 20 were negative for PSA expression. In the absence of PSA expression, we hypothesized that ADT may be of limited benefit. We identified four patients who were treated with ADT as first-line treatment for advanced disease. No PSA or radiographic responses were observed in any of the patients. This preliminary data suggest that these tumors may be androgen-independent and their growth not driven by the androgen receptor. Immunohistochemistry and RNA in situ hybridization studies looking at AR expression may help to better understand the lack of response observed in this small subset of tumors.
Consistent with sarcomatoid subtypes of other malignancies, metastatic sarcomatoid prostate cancer is associated with a poor prognosis.16, 17 Conventional chemotherapy and ADT did not appear to alter the natural course of the disease in this retrospective analysis. Different chemotherapy regimens (i.e. small cell carcinoma, sarcoma, prostate adenocarcinoma) were used to treat several patients with metastatic disease. Given the small number of patients, statistical analysis comparing each individual regimen is difficult to interpret. We observed short-lived, partial responses with both small cell and prostate cancer based regimens, but no durable responses were obtained. Further understanding of the tumor biology of sarcomatoid tumors and molecular profiling may identify targetable pathways and novel therapies for this aggressive neoplasm.
CONCLUSIONS
Localized, sarcomatoid prostate cancer can be effectively treated with definitive therapy including surgery and/or radiation. Favorable outcomes in the local disease group highlight the importance of early disease detection and treatment. More advanced disease, including bladder invasion or overt metastases confers a poor prognosis for survival. Moreover, our preliminary data from a small subgroup of patients showed no effect of ADT suggesting that tumor growth may not be dependent on AR signaling.
Acknowledgements
The project described was supported by ECOG Paul Carbone Fellowship and ASCO YIA, as well as by Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins NIH grants P30 CA006973. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None of the authors have potential conflicts of interest.
REFERENCES
- 1.Mazzucchelli R, Lopez-Beltran A, Cheng L, Scarpelli M, Kirkali Z, Montironi R. Rare and unusual histological variants of prostatic carcinoma: clinical significance. BJU international. 2008;102:1369–1374. doi: 10.1111/j.1464-410X.2008.08074.x. [DOI] [PubMed] [Google Scholar]
- 2.Tannenbaum M. Carcinoma with sarcomatoid changes or carcinosarcoma of prostate. Urology. 1975;6:91–93. doi: 10.1016/0090-4295(75)90603-2. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues DN, Hazell S, Miranda S, et al. Sarcomatoid carcinoma of the prostate: ERG fluorescence in-situ hybridization confirms epithelial origin. Histopathology. 2015;66:898–901. doi: 10.1111/his.12493. [DOI] [PubMed] [Google Scholar]
- 4.Leibovici D, Spiess PE, Agarwal PK, et al. Prostate cancer progression in the presence of undetectable or low serum prostate-specific antigen level. Cancer. 2007;109:198–204. doi: 10.1002/cncr.22372. [DOI] [PubMed] [Google Scholar]
- 5.Audet JF, Ruiz L, Sebe P, et al. Neoplasms induced by radiotherapy for prostate cancer: report of a case of pelvic sarcoma and review of the literature. Progres en urologie : journal de l'Association francaise d'urologie et de la Societe francaise d'urologie. 2004;14:420–422. [PubMed] [Google Scholar]
- 6.Canfield SE, Gans TH, Unger P, Hall SJ. Postradiation prostatic sarcoma: de novo carcinogenesis or dedifferentiation of prostatic adenocarcinoma? Techniques in urology. 2001;7:294–295. [PubMed] [Google Scholar]
- 7.Dominguez A, Piulats JM, Suarez JF, et al. Prostatic sarcoma after conservative treatment with brachytherapy for low-risk prostate cancer. Acta oncologica. 2013;52:1215–1216. doi: 10.3109/0284186X.2012.734927. [DOI] [PubMed] [Google Scholar]
- 8.Tseng TY, Sevilla DW, Moul JW, Maloney KE. Prostatic carcinosarcoma 15 years after combined external beam radiation and brachytherapy for prostatic adenocarcinoma: a case report. Prostate cancer and prostatic diseases. 2006;9:195–197. doi: 10.1038/sj.pcan.4500870. [DOI] [PubMed] [Google Scholar]
- 9.Hansel DE, Epstein JI. Sarcomatoid carcinoma of the prostate: a study of 42 cases. The American journal of surgical pathology. 2006;30:1316–1321. doi: 10.1097/01.pas.0000209838.92842.bf. [DOI] [PubMed] [Google Scholar]
- 10.Dundore PA, Cheville JC, Nascimento AG, Farrow GM, Bostwick DG. Carcinosarcoma of the prostate. Report of 21 cases. Cancer. 1995;76:1035–1042. doi: 10.1002/1097-0142(19950915)76:6<1035::aid-cncr2820760618>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. American journal of clinical oncology. 2009;32:27–29. doi: 10.1097/COC.0b013e31817b6061. [DOI] [PubMed] [Google Scholar]
- 12.Nazeer T, Barada JH, Fisher HA, Ross JS. Prostatic carcinosarcoma: case report and review of literature. The Journal of urology. 1991;146:1370–1373. doi: 10.1016/s0022-5347(17)38099-0. [DOI] [PubMed] [Google Scholar]
- 13.Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M. D. Anderson Cancer Center Experience. The Journal of urology. 2001;166:521–525. doi: 10.1016/s0022-5347(05)65974-5. [DOI] [PubMed] [Google Scholar]
- 14.Shannon RL, Ro JY, Grignon DJ, et al. Sarcomatoid carcinoma of the prostate. A clinicopathologic study of 12 patients. Cancer. 1992;69:2676–2682. doi: 10.1002/1097-0142(19920601)69:11<2676::aid-cncr2820691109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 15.Zizi-Sermpetzoglou A, Savvaidou V, Tepelenis N, Galariotis N, Olympitis M, Stamatiou K. Sarcomatoid carcinoma of the prostate: a case report. International journal of clinical and experimental pathology. 2010;3:319–322. [PMC free article] [PubMed] [Google Scholar]
- 16.Cheville JC, Lohse CM, Zincke H, et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. The American journal of surgical pathology. 2004;28:435–441. doi: 10.1097/00000478-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Yendamuri S, Caty L, Pine M, et al. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results Database analysis. Surgery. 2012;152:397–402. doi: 10.1016/j.surg.2012.05.007. [DOI] [PubMed] [Google Scholar]