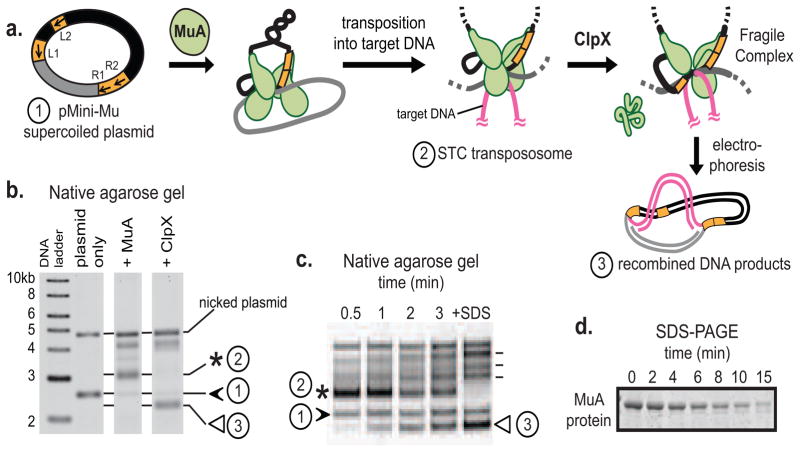
FIGURE 1. In vitro assays for MuA complex assembly and recognition by ClpX or ClpXP.
A) MuA transposase monomers and host protein HU (not depicted for clarity) are incubated with a supercoiled plasmid substrate (1, “pMini-Mu”) containing left and right phage Mu attachment sites (L1, L2, R1, R2 are sites of stable association in the assembled complex; other sites utilized during assembly but not bound by MuA in the final STC were omitted for clarity). MuA catalyzes DNA cleavage and recombination with target DNA to form the STC transpososome (2), a stable complex. ClpX remodels the transpososome by unfolding a subunit bound to L1 or R1 attachment site to produce the fragile complex. Unlike the stable STC, the fragile complex falls apart during gel electrophoresis and liberates recombined DNA products (3).
B) On a native agarose gel, supercoiled pMiniMu runs as a band between the 2 and 3 kb linear DNA markers (1, black arrow). In the “+MuA” lane, the STC transpososome appears as a band (2, asterisk) that migrates more slowly than supercoiled plasmid alone. Addition of ClpX to reactions containing STCs produces a set of recombined topoisomers; the lowest band (3, white arrow) was quantified for disassembly rates.
C) A timecourse shows ClpX catalyzed disassembly of transposomes. Rates of MuA complex disassembly by ClpX were assayed by measuring the rate of appearance of the lowermost disassembly DNA product (3, white arrow). Addition of SDS to the reaction disrupts all inter-protein and protein-DNA interactions within the STC transpososomes and serves as the “100% disassembly” control. Other recombined DNA products are indicated by dash lines next to +SDS lane; details on these topoisomers are in Maxwell et al. PNAS 1987.
D) A timecourse shows ClpXP catalyzed degradation of monomeric MuA. Rates of protein degradation were assayed by measuring the rate of disappearance of MuA on SDS-PAGE