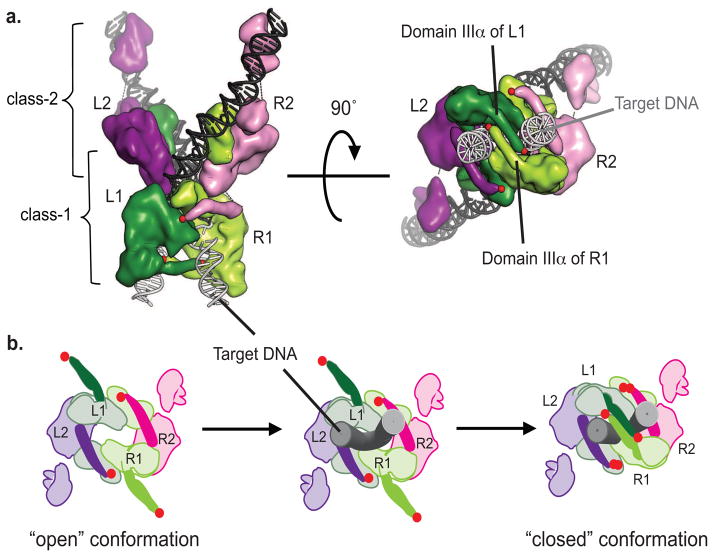
FIGURE 9. Model of conformational changes in MuA complex during transposition.
A) Structure-based cartoon of native STC (strand transfer complex); class-1 subunits in dark and light green (L1, R1), class-2 subunits in purple and pink (L2, R2), Mu DNA in black sticks, target DNA in grey sticks. Red dots indicate the C-terminal residue of domain IIIα. Side view on left and “bottom-up” view on right with target DNA coming out from page and Mu DNA facing into the page.
B) Diagram of “bottom-up” view of transpososome indicating proposed conformation changes to allow binding of target DNA. Color scheme same as in (A) with domain IIIα in solid colors and target DNA as gray tubes. After synapsing the left and right ends of the Mu genome, domain IIIa and IIIβ of both class-1 subunits must be in an “open” conformation to allow entry of target DNA brought by MuB. Then these domains swing into a “closed” conformation to trap the target DNA as observed in the crystal structure of the STC.