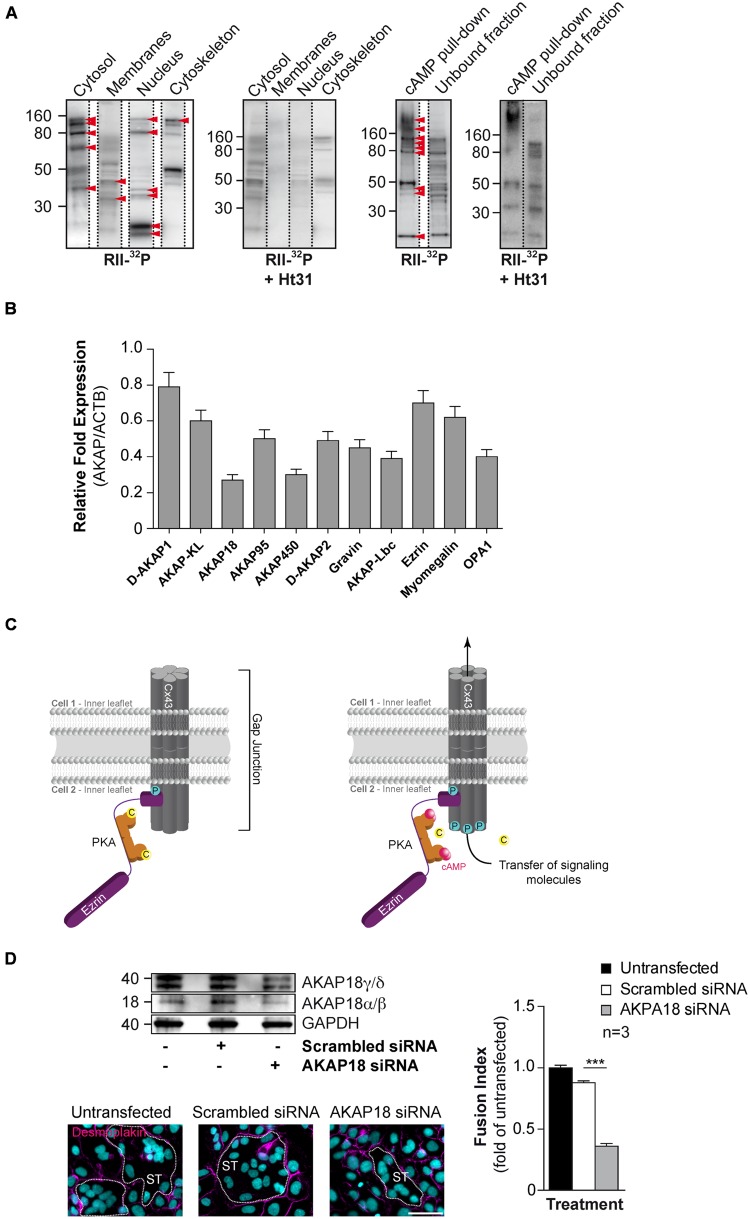
FIGURE 4.
Characterization of AKAPs in human CTs. (A) Proteins purified by sub-cellular fractionation (left) or by cAMP affinity chromatography (right) from cytrophoblasts were subjected to a solid phase binding assay using 32P-radiolabeled RII (RII-overlay) as a probe in the absence or presence of the Ht31 anchoring disruptor peptide (500 nM). Red arrowheads indicate putative AKAPs expressed in CTs. (B) Total RNA was purified from CTs (n = 3 cultures each) and subjected to RT-PCR with specific AKAP primers (identified in Table 1). Histograms represent mRNA expression of AKAPs in CTs normalized to beta-actin mRNA expression. (C) Cx43 gap junction communication is controlled by PKA anchoring through ezrin (Pidoux et al., 2014). Schematic depiction of a resting state gap junction in primary human trophoblast with Cx43 and a compartmentalized pool of PKA anchored to ezrin thus bound to Cx43 (left). Elevated intracellular cAMP levels lead to activation of PKA and subsequent spatiotemporally controlled phosphorylation of Cx43, which promotes the communication through gap junctions. This communication triggers trophoblast cell fusion. C, catalytic subunit of PKA; P for phosphorylation; pink dots, molecules of cAMP. (D) BeWo cells were transfected with AKAP18 siRNA (Invitrogen, Cat. # 1299001) or scrambled control, cultured for 48 h, stimulated for fusion with FSK (15 μM) for 24 h and subjected to immunoblot analysis with the indicated antibodies (upper left). Cells with AKAP18 knockdown or controls were stained for desmoplakin (magenta) and nuclei (DAPI). Syncytia (ST) boundaries are indicated by dashed lines. Scale bar: 30 μm. (Lower left) The effects of AKAP18 siRNA, scrambled control on fusion were assessed after 24 h treatment with FSK (15 μM) and summarized in histograms (right). Results are expressed as the mean ± SEM of n = 3 independent experiments (∗∗∗p < 0.001). Scale bar: 30 μm.