Figure 2.
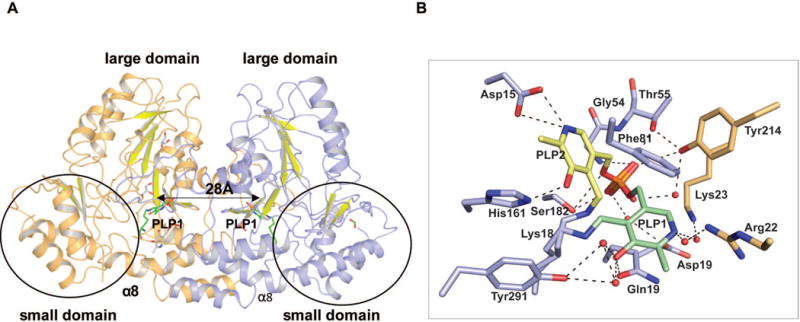
(A) Homodimer structure of AtmS13 (PDB 4ZWV). The two subunits are colored light orange and blue, respectively. The C-terminal, small domain is circled and the highlighted distance corresponds to distance between the respective active site bound cofactor pyridinium rings within the two monomers of the homodimer. (B) Residues involved in cofactor binding. The alternative PLP orientations (PLP1 and PLP2) are colored green and yellow, respectively. Active site residues are colored blue while the residues from the adjacent subunit contributing to the active site are colored orange.
