Abstract
Failure of natural killer (NK) cells or cytotoxic T-lymphocytes (CTL) to kill cognate target cells results in cytokine/chemokine hypersecretion and markedly delayed killer/target cell detachment. With congenital perforin deficiency, fatal cytokine storm results. In cancer cells, where corrupted apoptotic signaling frequently delays apoptosis, we propose that failed death may alter the tumor microenvironment and skew immune infiltrates, even when perforin is delivered normally.
Keywords: perforin, granzyme, inflammation, FHL, immunotherapy, detachment, apoptosis
As both NK cells and CTL can kill successive targets in rapid succession (‘serial killing’), the interaction is transient and the killer detaches from its ‘victim’ before engaging another. Decades ago, researchers observing this series of events aptly attached the term ‘kiss of death’ to this fatal interaction.1 Our study2 commenced out of our curiosity for how cell detachment occurs: we were aware that the literature on how CTL and NK cells form a stable conjugate boasts hundreds of papers, but there is virtually none that deals specifically with dis-engagement.
CTL and NK cells utilize very different receptors and co-receptors to recognize virus-infected or tumor target cells, but both need to form a stable conjugate to generate membrane signals that culminate in exocytosis of the potent granule-bound toxins, perforin and the serine protease granzymes (particularly granzyme B, GzmB) into the immune synapse.3 Perforin forms pore-like transmembrane channels that permit GzmB access to the target cell cytosol where caspase activation takes place. In humans and many types of outbred/wild mice, this occurs principally through the mitochondrial pathway due to direct truncation of Bid by GzmB; by contrast, the allotype of GzmB present in inbred mouse strains, GzmB predominantly processes pro-caspases directly.4,5
Our recent work is the first to shed some light on how CTL/NK cells terminate their contact with a target cell. Our findings arose from two sets of observations that we ultimately linked. Two years ago we developed live cell microscopy methodologies that pin-point the precise timing of the calcium flux that signals the CTL/NK cell is mobilizing its secretory granules to deliver perforin and GzmB to the immune synapse via exocytosis, a process also known as ‘degranulation’.6 By adding a higher than usual concentration (100µM) of the RNA-binding dye propidium iodide (PI) to the culture medium, we observed intense red fluorescence in the target cell some 60–80 s after degranulation in the killer. This signified that PI had entered the target cell cytosol via perforin pores that had rapidly assembled in the plasma membrane, a process we have shown to be unidirectional.7 Starting just 2-15 min later, the target cell started to show obvious apoptotic morphology, and detachment of the killer cell then followed.6
The second observation sprang from our interest in congenital perforin deficiency. Infants born with familial hemophagocytic lymphohistiocytosis Type 2 (FHL2) have bi-allelic mutations in the perforin (PRF1) gene, and die from an overwhelming cytokine storm soon after birth.8 To model the disorder, we allowed Prf1 gene-deficient mouse NK or TCR-transgenic CTL to form bona fide immune synapses under the time-lapse microscope, while also assaying the culture supernatant for inflammatory cytokines.
As we expected, the killer cells formed normal synapses, but failed to kill their targets. To our surprise, we also found that the two cells remained in contact for far longer (on average around five times as long) than when apoptosis was induced with wild type CTL/NK cells. Furthermore, calcium signaling in the Prf1-deficient effector lymphocytes did not terminate, with calcium levels continuing to oscillate indefinitely. As a result, secretion of the key cytokine interferon-γ and various chemokines was greatly enhanced in comparison to cultures that included immune-competent killers. The cytokine cocktail was able to induce naïve syngeneic macrophages to secrete large quantities of interleukin (IL)-6. These findings explained how the absence of perforin from lymphoid killer cells is linked to hyperactivation of the myeloid compartment, a deadly hallmark of FHL2 and related conditions.9,10
Studying rare but important diseases can be rewarding, but our study then took an unexpected twist with potentially far broader significance. NK and CTLs that expressed normal perforin but lacked granzymes also failed to killed target cells efficiently and showed a very similar phenotype to Prf1-null killers: delayed detachment, persistent calcium signaling and elevated interferon-γ secretion. Collectively, the results clearly indicated that detachment depended on the occurrence of target cell death, rather than the specific presence of either perforin or the granzymes.
In turn, this raised a key question: how is the signal for detachment generated? Does the killer cell possess a ‘molecular timer’ and detach after ‘sufficient’ time has elapsed for the target to be irreversibly damaged? Alternatively, does detachment occur only when the target cell ‘makes it known’ to the killer that cell death is proceeding, and its mission accomplished?
A very simple experiment showed the latter to be the case. When we reverted to wild type CTL or NK cells and delayed target cell death by blocking caspase activity (either chemically or by co-overexpressing Bcl–2 and XIAP), we were able to phenocopy perforin or granzyme deficiency. We are yet to precisely identify the signal for detachment, but our experiments show that a caspase-dependent event/s in the target cell is essential. Importantly, all our observations were reproduced in human NK and primary CTL clones as well as mouse CTL/NK cells, indicating the mechanism is conserved in evolution.
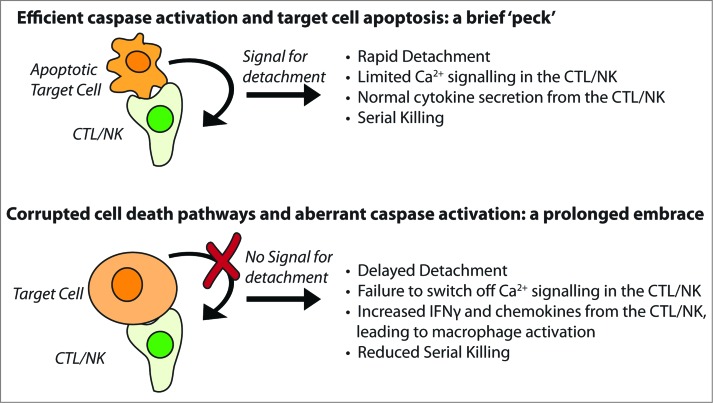
Primary immune deficiencies are all rare, but inhibition of cell death pathways is extremely common, both in cancer cells and cells infected with certain viruses. If our observations are valid in vivo, the array of cytokines and chemokines expressed by activated CTLs or NK cells in a tumor (or an infected organ) should depend on the degree to which target cell caspases can be activated (Fig. 1). It is easy to envisage that the composition of immune infiltrates might vary considerably (even in tumors of similar histological type), most obviously the degree and type of myeloid cell infiltration and activation.
Figure 1.
The ‘kiss of death’ can vary in length, intensity and consequence. Wild type CTL or NK cell (top panel) deliver perforin and granzymes and kill target cell by caspase-dependent apoptosis. In this instance, the immune synapse is short lived, as a caspase-dependent signal indicates that cell death is inevitable, and the killer cell detaches in search of a further target. When the killer cell has impaired perforin delivery or function (such as in FHL2), or caspases are not efficiently activated (bottom panel), the cells remain in contact and inflammatory cytokines are secreted from CTL/NK in abundance.
After 40 years, our work has shown that the ‘kiss of death’ can take different forms. Typically, a quick ‘peck’ is sufficient to dispatch the target cell. But if there is significant resistance on the part of the victim, the ‘kiss of death’ can transform into a prolonged involuntary embrace with sinister sequelae.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ginsburg H, Ax W, Berke G. Graft reaction in tissue culture by normal rat lymphocytes. Transplant Proc 1969; 1:551-5; PMID:5406839 [PubMed] [Google Scholar]
- 2.Jenkins MR, Rudd-Schmidt JA, Lopez JA, Ramsbottom KM, Mannering SI, Andrews DM, Voskoboinik I, Trapani JA. Failed CTL/NK cell killing and cytokine hypersecretion are directly linked through prolonged synapse time. J Exp Med 2015; 212:307-17; PMID:25732304; http://dx.doi.org/ 10.1084/jem.20140964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez JA, Brennan AJ, Whisstock JC, Voskoboinik I, Trapani JA. Protecting a serial killer: pathways for perforin trafficking and self-defence ensure sequential target cell death. Trends Immunol 2012; 33:406-12; PMID:22608996; http://dx.doi.org/ 10.1016/j.it.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 4.Kaiserman D, Bird CH, Sun J, Matthews A, Ung K, Whisstock JC, Thompson PE, Trapani JA, Bird PI. The major human and mouse granzymes are structurally and functionally divergent. J Cell Biol 2006; 175:619-30; PMID:17116752; http://dx.doi.org/ 10.1083/jcb.200606073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andoniou CE, Sutton VR, Wikstrom ME, Fleming P, Thia KY, Matthews AY, Kaiserman D, Schuster IS, Coudert JD, Eldi P et al.. A natural genetic variant of granzyme B confers lethality to a common viral infection. PLoS Pathog 2014; 10:e1004526; PMID:25502180; http://dx.doi.org/ 10.1371/journal.ppat.1004526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez JA, Susanto O, Jenkins MR, Lukoyanova N, Sutton VR, Law RH, Johnston A, Bird CH, Bird PI, Whisstock JC et al.. Perforin forms transient pores on the target cell plasma membrane to facilitate rapid access of granzymes during killer cell attack. Blood 2013b; 121(14):2659-68; PMID:23377437; http://dx.doi.org/ 10.1182/blood-2012-07-446146 [DOI] [PubMed] [Google Scholar]
- 7.Lopez JA, Jenkins MR, Rudd-Schmidt JA, Brennan AJ, Danne JC, Mannering SI, Trapani JA, Voskoboinik I. Rapid and unidirectional perforin pore delivery at the cytotoxic immune synapse. J Immunol 2013a; 191:2328-34; PMID:23885110; http://dx.doi.org/ 10.4049/jimmunol.1301205 [DOI] [PubMed] [Google Scholar]
- 8.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, Henter JI, Bennett M, Fischer A, De Saint Basile G et al.. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science 1999; 286:1957-9; PMID:10583959; http://dx.doi.org/ 10.1126/science.286.5446.1957 [DOI] [PubMed] [Google Scholar]
- 9.Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med 2012; 63:233-46; PMID:22248322; http://dx.doi.org/ 10.1146/annurev-med-041610-134208 [DOI] [PubMed] [Google Scholar]
- 10.De Saint Basile G, Menasche G, Fischer A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat Rev Immunol 2010; 10:568-79; PMID:20634814; http://dx.doi.org/ 10.1038/nri2803 [DOI] [PubMed] [Google Scholar]