Abstract
Context:
Chronic inflammation may increase the risk of fracture, and omega-3 polyunsaturated fatty acids (PUFAs) may reduce fracture risk via down-regulation of inflammatory cytokine gene expression and other mechanisms.
Objective:
We investigated associations between baseline samples of inflammatory markers, TNFα soluble receptors 1 and 2 (TNFα-sR1 and -sR2), and incident hip fracture. These associations were then tested for effect modification by dietary PUFA intake estimated by a baseline food frequency questionnaire.
Design and Setting:
A nested case-control study was conducted among participants of the Women's Health Initiative Observational Study (ages, 50–79 y). Multivariable conditional logistic regression models were constructed to account for the paired design.
Participants:
This study sampled 400 pairs of hip fracture cases and controls without incident hip fracture, matched on age, year of enrollment, and menopausal hormone use.
Main Outcome Measures:
Odds ratio of hip fracture by quartile of TNF soluble receptors.
Results:
The odds ratio of hip fracture comparing the highest to lowest quartiles was 2.24 (95% confidence interval, 1.05–4.79; P for linear trend, .048) for TNFα-sR1 and 2.83 (95% confidence interval, 1.34–5.99; P for linear trend, .011) for TNFα-sR2, adjusted for FRAX hip fracture score, nutritional variables, and selected factors impacting inflammation; there was a gradient of risk by increasing quartile in TNFα-sR1. PUFA intake did not modify these associations.
Conclusions:
Women with the highest levels of TNFα-sR1 and TNFα-sR2 had a greater than 2-fold increased hip fracture risk, independent of other fracture risk factors. These associations did not differ by high vs low PUFA intake.
Hip fracture constitutes a major public health problem (1) with annual incidence projected to increase 50% to approximately 450 000 fractures by 2025 in the United States (2). The annual direct cost of hip fracture, $12 billion in 2005, is estimated to increase 49% by 2025. Declines in functional status after hip fracture include disabilities in walking, transferring, and grooming (3). Excess mortality is higher, not only within the first year, and persists more than 2-fold higher up to 10 years after the index fracture (4, 5).
Chronic low-grade systemic inflammation may contribute to the pathogenesis of osteoporosis, a major risk factor for hip fracture. TNFα is an inflammatory cytokine that stimulates osteoclastogenesis in vivo via increasing responsiveness to receptor activator of nuclear factor-κB ligand (6, 7). TNFα also induces vascular endothelium to increase adhesion and transmigration of preosteoclasts, thereby recruiting the latter cells to sites of inflammation (8). Higher quartiles of baseline TNFα levels were associated with increased incidence of total fracture in a cohort study enrolling 70- to 79-year-old women and men, but this did not reach statistical significance in adjusted models (9). Levels of serum TNFα, as a biomarker for fracture, may be limited by its relatively short half-life and large variability within the population (10). TNFα soluble receptors 1 and 2 (TNFα-sR1 and TNFα-sR2) have been identified in human serum, are shed along with TNFα, and offer a more stable measure of long-term exposure to TNFα (11, 12). TNF soluble receptors may function as a TNFα antagonist by binding the cytokine, thus reducing inflammation; however, soluble receptors may also preserve the bioactive trimeric form of TNFα and potentially serve as a reservoir for TNFα as the latter dissociates from the receptor-ligand complex. Recently, TNFα-sR1 and TNFα-sR2 have been associated with increased risk of total fracture and hip fracture in observational studies (9, 13, 14).
Dietary intake of certain fatty acids has also been associated with hip fracture risk in large epidemiological studies (15, 16). Higher saturated and lower monounsaturated fatty acid intakes as measured by a baseline food frequency questionnaire (FFQ) were associated with higher incident hip fracture risk in a previous analysis of Women's Health Initiative (WHI) data (15). In a Framingham Study cohort of older adults, higher consumption of specific omega-3 (n-3) and omega-6 (n-6) polyunsaturated fatty acids (PUFAs) was associated with reduced hip fracture risk (16). Similarly, higher red blood cell (RBC) levels of total n-3 PUFA, specific n-3 PUFAs (α-linoleic acid [ALA] and eicosapentaenoic acid [EPA]), and a lower n-6/n-3 ratio were each associated with lower incident hip fracture risk in a nested case-control study within the WHI (17). The n-3 and n-6 PUFAs are particularly interesting in relation to bone metabolism because of their ability to modulate inflammatory processes. Peripheral mononuclear cell cultures supplemented with EPA and docosahexaenoic acid (DHA) decreased production of TNFα and IL-6 (18). The mechanisms by which n-3 PUFAs exert their effects on bone are not clear but may include modulation of transcription factors involved in bone turnover (19), stem cell differentiation (20–22), and calcium absorption or excretion (23), as well as dampening inflammation.
We therefore examined data from the WHI using a separate set of cases and controls to confirm the association of soluble TNF receptors and incident hip fracture using a nested case-control design. We then tested whether dietary fatty acid intakes might modify these associations.
Subjects and Methods
Study population
The WHI enrolled 161 808 women (age, 50–79 y) from 1993–1998 at 40 US clinical centers as previously reported (24). Within the WHI, the Observational Study (WHI-OS) enrolled 93 676 women (age, 50–79 y) from 1994–1998 at 40 clinical centers throughout the United States in a prospective cohort to explore factors affecting morbidity and mortality in women as they age. The WHI-OS women were eligible if they were postmenopausal, unlikely to move or die within 3 years, ineligible or not interested in WHI-Clinical Trials (WHI-CT) participation, and not currently participating in any other clinical trial. The Institutional Review Board at each of the participating centers approved the study, and all participants provided written informed consent.
Selection of cases and controls
The study population was restricted to Caucasian women who had been part of a previous whole genome association study. From this group, 400 randomly selected incident cases of hip fracture and their matched controls were identified for this nested case-control study. Controls were selected using random sampling in a 1:1 ratio with cases from the subpopulation of women who reported no prevalent postmenopausal fractures (no self-reported fracture at age ≥ 55 y) at baseline and no incident hip fracture through the planned study closeout (March 31, 2005). Individual matching was done by age (±1 y), enrollment date (±1 y), and current menopausal hormone therapy (HT) use at baseline (exact). Matching by enrollment date served the dual purpose of matching on length of follow-up and length of frozen storage for the serum specimens. Exclusion criteria included prior therapy with a bisphosphonate or other bone-active medication (calcitonin, raloxifene, tamoxifen, and teriparatide), as well as missing information on hormone use at baseline (to limit missing data). Postmenopausal prevalent fractures were excluded in controls due to concerns that some of the pathways associated with hip fracture risk might overlap those associated with risk for total fractures.
Follow-up and fracture ascertainment
Median time to hip fracture was 5.0 years (range, 0.1–9.4 y) as of March, 2005, and median follow-up time for controls was 8.7 years (range, 3.6–10.5 y). The outcome variable, incident hip fracture, was self-reported and centrally adjudicated by trained physicians reviewing medical records including radiology reports as previously described (25). High vs low trauma was not distinguished.
Laboratory assessment
Serum TNFα-sR1 and TNFα-sR2 concentrations were measured from samples obtained at the baseline visit (1993–1998). Participants were instructed to take all usual medication except for diabetes medications, abstain from smoking for at least 1 hour, refrain from vigorous physical activity for at least 12 hours, hold aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) for 48 hours, and fast for 12 hours before study visit. Phlebotomy was performed in a seated position; samples were processed locally and stored at −70°C until shipment on dry ice to a central site (McKesson Bioservices, Rockville, MD), where they were maintained at −80°C for long-term storage until assay (26). Measurement of serum TNFα-sR1 and TNFα-sR2 concentrations was performed at the University of Vermont in duplicate using ELISA (R&D Systems). Detectable limits for TNFα-sR1 using a DRT100 kit and TNFα-sR2 using a DRT200 kit were 3 and 1 pg/mL, respectively. Interassay coefficients of variation were 6.7–10% and 5.6–6.2%, respectively. Laboratory personnel were blinded to case-control status.
Covariate assessment
Covariates were obtained from screening questionnaires and interviews at the baseline enrollment, including data on demographics, medical history, medications, and lifestyle factors, including predictors of hip fractures based on Robbins et al (27). The 10-year absolute risk of hip fracture without bone mineral density was estimated by FRAX score, version 3.0 (28). Factors that impact systemic inflammation were also collected, including NSAID use, history of arthritis, depressive symptoms, body mass index (kilograms/meter2) calculated from measured height and weight, self-reported general health, comorbidity index, slowness/weakness, poor endurance/exhaustion, and physical activity. A modified Charlson comorbidity index (range, 0–15) was used based on the study of Gold et al (29). We adjusted for frailty, using 3 components of the WHI frailty index per Woods et al (30): slowness/weakness (using Rand SF-36 Physical Function Scale; range, 0–100), poor endurance/exhaustion (Rand SF-36 Vitality Scale: range, 0–100), and usual physical activity level (metabolic equivalent hours per week [MET-h/wk]). Cut-points based on previously described lowest quartiles were used for the Physical Function Scale, Vitality Scale, and physical activity to indicate frailty: <75, <55, and ≤ 3, respectively (30). Lower values for this modified frailty index indicate greater frailty.
Nutritional variables were assessed by semiquantitative FFQ at the baseline visit. The FFQ contained 122 questions on food items/groups, 19 questions to determine fat intake more precisely, and four summary questions assessing usual intake of fruits, vegetables, and added fats (31). The University of Minnesota's Nutrition Coordinating Center database (version 30; Minnesota Nutrition Data System for Research) was used to derive nutrient intakes (32). We extracted data on intake of calcium (diet and supplement), vitamin D (diet and supplement), total calories, and major food categories of carbohydrate, protein, and fat. Fat intake was further divided into saturated, monounsaturated, and polyunsaturated fats. Using a nutrient density approach, individual nutrients were expressed as percentages of total kilocalorie intake (% kcal).
Candidate effect modifiers were those dietary fatty acid components showing an association with TNFα receptors. Plant-based (ALA) vs marine-derived (EPA + DHA) n-3 PUFAs were assessed separately because these had separate effects on incident total fracture risk (15). Only total n-6 PUFA was assessed because the vast majority of n-6 is consumed in the form of linoleic acid (LA). Dietary fatty acids correlated with TNF receptors were individually added to the model to assess for the presence of effect modification.
Statistical analysis
Baseline subject characteristics and dietary intake were compared between cases and controls by McNemar's test for categorical variables and by paired t-tests and Wilcoxon signed-rank test for continuous variables, respectively. Multivariable statistical models were constructed using conditional logistic regression, accounting for paired design. TNFα-sR1 and TNFα-sR2 values were categorized by quartiles, with cut-points based on the distribution of values within controls, who better represent the distributions of these exposures within the general population from which cases derive. Odds ratios (ORs) with 95% confidence intervals (CIs) for hip fracture were estimated by incremental quartile of TNFα receptor vs quartile 1 as the referent group. Fracture risk by FRAX score (28) was added to the base model (TNFα receptor, only), followed by the addition of covariate data on nutritional variables and selected factors impacting inflammation. Tests of trend were performed across increasing quartiles of TNFα receptors to assess for a dose-response relationship with hip fracture. Associations of PUFA intake and TNFα receptors were assessed using Spearman correlations. Finally, various dietary fatty acid intakes were added to the fully adjusted model to assess for the presence of effect modification, including main effect and interaction terms of TNFα receptor quartile by fatty acid. The significance level for all analyses was P < .05.
Results
Subject characteristics and dietary intake
The baseline mean ± SD age of subjects was 70 ± 6.4 years. All subjects were postmenopausal Caucasian women (Table 1). One-third (n = 133) of hip fracture cases reported a personal history of fracture, vs none for the controls. Cases had higher FRAX scores than controls and were more likely to report a personal history of fracture, current corticosteroid use, current cigarette smoking, and less physical activity, and they were lighter and had lower body mass index values. Cases had greater indices of frailty with poorer self-reported general health, greater comorbidity, poorer physical function, and poorer endurance/exhaustion. The two groups did not differ with respect to parental history of fracture, alcohol use, treated diabetes, rheumatoid arthritis, depressive symptoms, arthritis, or NSAID use. Likewise, dietary intake did not differ between cases and controls for calcium, vitamin D, total calories, carbohydrate, protein, and fat—including polyunsaturated, total n-6 fatty acids, LA, arachidonic acid, total n-3 fatty acids, ALA, EPA, and DHA (Table 2). Cases reported higher saturated and monounsaturated fat intake compared with controls; but mean differences were modest, <1% of kcal, so these variables were not entered into multivariate models for adjustment.
Table 1.
Baseline Characteristics of Hip Fracture Cases and Controls from the WHI-CT and WHI-OS
| Variable | Cases (Hip Fracture) | Controls (No Hip Fracture) | P |
|---|---|---|---|
| White race/ethnicity | 400 (100) | 400 (100) | 1.00 |
| Age at screening, mean (SD), y | 69.8 (6.4) | 69.9 (6.4) | .67 |
| Personal history of fracture age ≥55 ya | 113 (33.1) | 0 (0) | c |
| Parental history of fracturea | 161 (43.3) | 157 (41.5) | .71 |
| Current HT use | 151 (37.8) | 151 (37.8) | 1.00 |
| Current corticosteroid use | 13 (3.3) | 4 (1.0) | .049 |
| Current smoker | 35 (8.8) | 15 (3.8) | .0045 |
| Current alcohol intakea | 245 (70.2) | 277 (76.5) | .10 |
| Current NSAID use | 81 (20.3) | 73 (18.3) | .52 |
| Treated diabetes | 34 (8.5) | 21 (5.3) | .10 |
| Rheumatoid arthritis | 27 (6.9) | 16 (4.1) | .081 |
| Arthritisa | 237 (59.8) | 207 (52.7) | .054 |
| Depressive symptoms (score)a | 0.053 (0.15) | 0.043 (0.14) | .31 |
| Height, cma | 161.8 (6.6) | 160.6 (6.3) | .0082 |
| Weight, kga | 67.3 (14.6) | 70.3 (17.4) | .0088 |
| Body mass index, kg/m2a | 25.6 (5.0) | 27.0 (5.8) | .0003 |
| Very good-excellent general healtha | 134 (39.4) | 219 (59.2) | <.0001 |
| Comorbidity indexa | 1.01 (1.15) | 0.70 (1.06) | .0004 |
| Physical functiona | 167 (42.8) | 92 (23.6) | <.0001 |
| Poor endurance/exhaustiona | 147 (36.9) | 90 (22.6) | <.0001 |
| Physical activity, MET/wka | 10.9 (10.9) | 14.6 (14.6) | <.0001 |
| FRAX score (10-y hip fracture % risk) | 7.4 (6.0) | 5.3 (5.9) | <.0001 |
| TNFα-sR1, pg/mLa,b | 1547 (498) | 1467 (494) | .0004 |
| TNFα-sR2, pg/mLa,b | 3243 (927) | 3119 (834) | .0007 |
Data are expressed as number (percent) unless specified otherwise. P values are from paired t-tests and Wilcoxon signed-rank test for continuous variables or McNemar's test for dichotomous variables.
Sample sizes for those variables are between 328 and 399 due to missing data; sample sizes are 400 otherwise.
Median and interquartile range.
This variable could not be tested due to lack of controls with personal history of fracture.
Table 2.
Dietary Intakes of Hip Fracture Cases and Controls from the WHI-CT and WHI-OS
| Dietary Variable | Cases (Hip Fracture) | Controls (No Hip Fracture) | P |
|---|---|---|---|
| Total energy intake, kcal | 1488 (680) | 1451 (554) | .39 |
| Carbohydrate, % kcal | 51.8 (9.6) | 52.9 (9.5) | .15 |
| Protein, % kcal | 16.7 (3.4) | 16.9 (3.1) | .48 |
| Fat, % kcal | |||
| Saturated fat | 10.6 (3.6) | 10.0 (3.5) | .02 |
| Monounsaturated fat | 11.8 (3.7) | 11.2 (3.4) | .05 |
| Polyunsaturated fat | 6.5 (2.1) | 6.3 (2.1) | .19 |
| n-6 fatty acid | 5.7 (1.9) | 5.5 (1.9) | .17 |
| LA | 5.7 (1.9) | 5.5 (1.9) | .17 |
| AA | 0.05 (0.03) | 0.05 (0.02) | .34 |
| n-3 fatty acids | 0.8 (0.3) | 0.8 (0.3) | .62 |
| ALA | 0.7 (0.3) | 0.7 (0.3) | .37 |
| EPA + DHA | 0.07 (0.06) | 0.07 (0.07) | .13 |
| Calcium, mg/1000 kcala | 849 (575) | 906 (539) | .15 |
| Vitamin D, μg/1000 kcala | 7.2 (6.1) | 7.3 (5.7) | .92 |
Abbreviation: AA, arachidonic acid. Hip fracture values for cases and controls are expressed as % kcal (SD) unless specified otherwise; n = 400 for cases, n = 400 for controls. P values are from paired t-tests for continuous variables.
Values are from diet plus supplement intake.
TNF receptors and hip fracture
Baseline serum TNFα-sR1 concentrations were higher in cases than controls (P = .0004), as were TNFα-sR2 concentrations (P = .0007) (Table 1). In models for TNFα-sR1 using Q1 as the referent stratum (OR = 1) and matched for age, race, year of enrollment, and menopausal HT (Table 3, model 1), hip fracture ORs for Q2, Q3, and Q4 were each statistically significant, with the highest risk in Q4 vs Q1: OR = 2.27 (CI, 1.45–3.55), P = .0003 (Table 3, model 1). For TNFα-sR2, there was an increasing gradient of risk, with OR of hip fracture increasing from 1.41 to 2.10, but the risk was significant only for Q4 vs Q1: OR = 2.10 (CI, 1.36–3.24), P = .0008 (Table 3, model 1). A linear trend test showed increasing ORs for hip fracture with higher quartiles of TNF receptors: each higher quartile of TNFα-sR1 and TNFα-sR2 was associated with 25.7% (P = .0011) and 22.8% (P = .0010) greater risk of hip fracture, respectively.
Table 3.
Multivariable Adjusted OR of Hip Fracture Based on Quartiles of TNF-sR1 and TNFsR2
| n | Q1a | Q2a | Q3a | Q4a | P for Trend | |
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| TNFα-sR1 | 396 | 1 | 1.77* (1.14, 2.74) | 1.67* (1.09, 2.56) | 2.27*** (1.45, 3.55) | .0011 |
| TNFα-sR2 | 391 | 1 | 1.41 (0.91, 2.20) | 1.48 (0.94, 2.31) | 2.10*** (1.36, 3.24) | .0010 |
| Model 2 | ||||||
| TNFα-sR1 | 396 | 1 | 1.74* (1.07, 2.82) | 1.86* (1.15, 2.98) | 3.11*** (1.87, 5.17) | <.0001 |
| TNFα-sR2 | 391 | 1 | 1.70* (1.03, 2.79) | 1.61 (0.97, 2.65) | 2.76*** (1.68, 4.51) | .0001 |
| Model 3 | ||||||
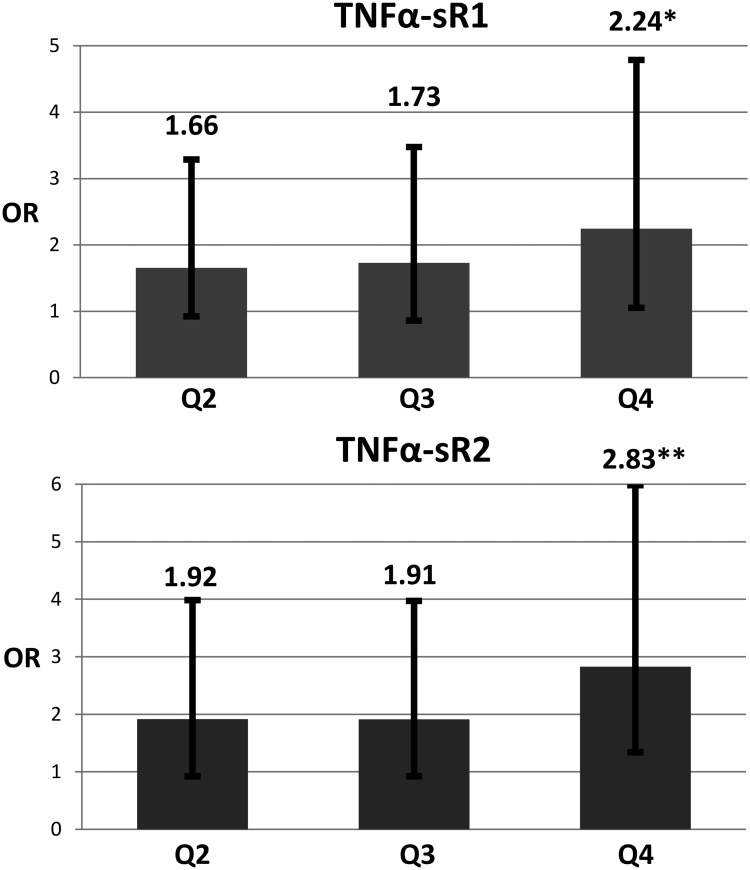
| TNFα-sR1 | 250 | 1 | 1.66 (0.84, 3.29) | 1.73 (0.86, 3.48) | 2.24* (1.05, 4.79) | .0475 |
| TNFα-sR2 | 245 | 1 | 1.92 (0.92, 3.98) | 1.91 (0.92, 3.97) | 2.83** (1.34, 5.99) | .0113 |
Data are expressed as OR (95% CI) obtained from logistic regression models. Quartile 1 is the reference stratum (OR = 1). “n” represents the number of case and control pairs used in the analysis; it varies due to missing covariate data. Model 1 is matched on age, race, year of enrollment, self-reported hormone use; no adjustment for other covariates. Model 2 is model 1 plus FRAX score. Model 3 is model 2 plus nutritional variables (intake of calcium, vitamin D, total energy, protein, multivitamin use), and factors impacting inflammation (NSAID use, arthritis, depression, body mass index, self-reported general health, comorbidity index, physical function, poor endurance/exhaustion, physical activity).
Interquartile cut-points for TNFα-sR1: 1246.0, 1465.2, and 1739.4 pg/mL; TNFα-sR2: 2674.4, 3118.4, and 3509.5 pg/mL (using controls only).
P < .05 relative to Q1;
P < .01;
P < .001. P values are from tests for linear trend.
After adjustment for hip fracture risk by FRAX score (Table 3, model 2), TNF soluble receptors remained predictors of hip fracture, and the ORs generally increased in magnitude compared to those in model 1. For TNFα-sR1, the hip fracture OR for Q2, Q3, and Q4 vs Q1 remained statistically significant, now with an increasing gradient of risk. For TNFα-sR2, adjustment for FRAX score increased the point estimates of OR for each quartile above those in the base model to 1.66–2.76 (9–31% increases), and the Q2 vs Q1 comparison became significant. Tests of linear trend by quartile of TNFα-sR1 and TNFα-sR2 remained significant (P < .0001 and P = .0001, respectively).
Effect modification by PUFA
Intake levels of EPA, DHA, and EPA + DHA were each inversely associated with TNFα-sR1 and TNFα-sR2 (all P < .001). Spearman correlations of TNFα-sR1 and TNFα-sR2 with EPA were −0.12 and −0.13; DHA, −0.13 and −0.14; and EPA + DHA, −0.13 and −0.13, respectively. ALA, SFA, MUFA, and PUFA did not show significant associations with either TNFα receptor. Before investigating effect modification by fatty acid, five nutritional variables and nine select factors impacting inflammation were added to the model (Table 3, model 3; Figure 1). OR reached statistical significance only in the Q4 vs Q1 comparison for both TNF receptors. Tests of linear trend showed increases of 27.4 and 35.5% with each higher quartile of TNFα-sR1 and TNFα-sR2, respectively (P = .048 and P = .011, respectively).
Figure 1.
OR of hip fracture by quartiles of TNF-sR1 and TNF-sR2. Quartile 1 is reference strata; OR = 1. Linear trend: *, P < .05; **, P < .01. Error bars denote 95% CI. Data are adjusted for FRAX score, nutritional variables (intake of calcium, vitamin D, total energy, protein, multivitamin use), and factors impacting inflammation (NSAID use, arthritis, depression, body mass index, self-reported general health, comorbidity index, physical function, poor endurance/exhaustion, and physical activity).
EPA, DHA, and EPA + DHA intake levels were then individually added into the fully adjusted model to assess for evidence of effect modification. All main effects and interaction terms were nonsignificant except for total PUFA × TNFα-sR2 Q2 (P = .02). We doubted the validity of this apparent statistical interaction because both individual terms were nonsignificant and the point estimate in the model was unrealistically large (48.3), and we concluded that there was a lack of evidence for effect modification.
Discussion
Data from this prospective nested case-control study within the WHI show that in postmenopausal Caucasian women, higher baseline TNFα-sR1 and TNFα-sR2 levels were associated with greater incident hip fracture risk over 8.7 years of follow-up. The predictive ability of these inflammatory biomarkers was independent of the estimated 10-year hip fracture risk by FRAX score, nutritional variables, and selected factors impacting inflammation and was not modified by fatty acid intakes. To place the magnitude of risk within a clinical context, we also ran models testing the association of TNF receptors as a continuous predictor of hip fracture risk using log base 0.2 of TNF receptors. In models adjusting for FRAX score, a 20% greater TNFα-sR1 and TNFα-sR2 is associated with a greater OR of hip fracture by 27.5% (95% CI, 17.3–47.9; P < .0001) and 33.0% (95% CI, 14.8–54.2; P = .0002).
The current study lends further support to the ability of circulating inflammatory biomarkers to predict osteoporotic fracture. Cauley et al (9) assessed whether a panel of baseline inflammatory markers—IL-6, TNF-α, C-reactive protein, and soluble receptors of IL-6, IL-2, TNFR1, TNFR2—could predict incident total fracture within the Health ABC study, a prospective cohort study of > 3000 healthy adults, ages 70–79 years. Multivariate-adjusted hazard ratios (95% CI) of total fracture in Q4 vs Q1–Q3 for IL-2sR, TNFα-sR1, and TNFα-sR2 were 1.52 (1.04–2.21), 1.73 (1.18–2.55), and 1.48 (1.01–2.20), respectively. Due to the small numbers of hip fractures, there was limited power to test these biomarkers for this outcome. Building on the hypothesis, Barbour et al (13) focused on cytokine soluble receptors, including TNFR1 and TNFR2, as predictors of hip fracture within the WHI-OS using a nested case-control design. The highest quartiles of soluble receptors were compared with the lower three quartiles combined to show statistically significant risk ratios for TNFα-sR2 [1.56 (1.09–2.22)] but not TNFα-sR1 [1.41 (0.97–2.05)]. Models incorporating multiple inflammatory markers (TNFα-sR1, TNFα-sR2, and IL-6sR) showed that the presence of three markers in the highest quartile predicted hip fracture. In the Study of Osteoporotic Fracture (SOF), the highest quartiles of TNFα-sR1 and IL-6 were associated with hip fracture (hazard ratio = 2.05; 95% CI, 1.35–3.12; and hazard ratio = 1.64; 95% CI, 1.09–2.48, respectively) (14).
Although the current and previous studies (13, 15) are nested case-control studies within the WHI, it should be noted that each was a separate sampling of hip fractures. The strength of association between soluble TNF receptors and hip fracture is greater in the present study. Here, ORs (Q2, Q3, and Q4 vs Q1) for TNFα-sR1 (1.66, 1.73, 2.24) and TNFα-sR2 (1.92, 1.91, 2.83) are greater than the comparable point estimates in the prior study (1.00, 1.14, 1.48; and 1.09, 0.96, 1.57, respectively) (13). Although Barbour et al (13) reported risk ratios and the current paper expresses ORs, the ORs are comparable due to the low frequency of hip fracture. Annual rates of hip fracture have been reported at 0.16% in the WHI-OS and 0.16% in the WHI-CT (27).
The greater effect size of TNFα receptors as a predictor of hip fracture in the current vs prior study may be due to subtle differences in population characteristics. Interquartile cut-points for TNFα-sR1 among controls were lower in the current study (1246.0, 1465.2, and 1739.4 pg/mL) compared to the values found in the prior WHI study (13) (1373.3, 1566.7, and 1842.7 pg/mL), suggesting that inflammation was higher overall in the latter study. Lower referent quartile values in the current study could have resulted in higher risks in the higher quartiles. However, the opposite situation occurred for TNFα-sR2 interquartile cut-points, which were higher in the current study (2674.4, 3118.4, and 3509.5 pg/mL) vs the prior study (2113.5, 2489.7, and ≥ 2848.4). In the current study, almost 40% of women took estrogen, and personal history of fracture in control participants was excluded by design. In contrast, Barbour et al (13) excluded participants using estrogen up to 1 year before enrollment and included personal history of fracture in controls. Subjects in the current study vs the prior study had greater physical activity (10.9–14.6 vs. 7–11 MET-h/wk). Finally, time to hip fracture was shorter in the current study vs the prior study (median, 5.1 vs 7.1 y), suggesting that the relationship may attenuate with time.
Three possible relationships between PUFA and hip fracture are considered: 1) PUFAs are in the causal pathway involving inflammation (PUFA→TNF receptors→fracture); 2) PUFAs affect hip fracture risk via other mechanisms; and 3) the PUFA effect modifies the relationship between inflammation and hip fracture. We found evidence of the first relationship because EPA, DHA, and EPA + DHA were inversely associated with both soluble TNF receptors, and the latter predict hip fracture. However, the association between PUFAs and hip fracture without adjustment for TNFα receptors was nonsignificant in this sample. Therefore, we did not perform mediation analysis testing. Regarding the second relationship, cohort studies assessing fatty acid intake by FFQ, lower saturated and higher monounsaturated fat intakes, and higher ALA were associated with lower hip fracture risk from the WHI and Framingham studies, respectively (15, 16), and in our prior work higher baseline RBC levels of ALA, EPA, and total n-3 fatty acids and lower n-6/n-3 ratio were each associated with lower hip fracture (17). In the current study, ORs of hip fracture by these PUFAs were not statistically significant. Although PUFAs may impact hip fracture via lowering inflammation or via another mechanism(s), it is unlikely that PUFAs are the sole determinants of soluble TNFα receptor levels. Because there are many likely determinants of chronic inflammation, the addition of PUFA as a possible effect modifier was legitimate. In this study, we searched for effect modification by PUFA intake but did not find evidence of such.
Study strengths include the nested case-control study design within a prospective study, rigorous adjudication of hip fractures, and broad covariate data, including those associated with inflammation: comorbidity index, frailty, and physical function. Potential limitations include the subjective assessment of nutrient intake via FFQ and fluctuation of TNFα receptor levels after baseline determination. We preferred to measure tissue levels of RBC PUFA directly, but there was limited availability of samples containing both these and soluble TNFα receptors. Because this study was limited to postmenopausal Caucasian women, results may not be generalizable to men, non-Caucasians, or premenopausal women. Renal function measured by cystatin-C and bone mineral density attenuated the ability of inflammatory markers to predict hip fracture risk in the SOF and WHI but were not assessable in the current study. Another limitation was our inability to capture n-3 supplement use in WHI participants. Finally, causality may not be inferred from this association based on a case-control study.
It is possible that PUFAs do modulate the relationship between TNF receptors and hip fracture, but this may require supplementation to reach levels higher than achieved by diet alone. One ancillary study within the Vitamin D and Omega-3 Trial (VITAL)—a randomized, double-blind, placebo-controlled, 2 × 2 factorial trial of EPA + DHA (1:3 to 1 ratio), 1 g/d and vitamin D3, 2000 IU/d in 20 000 US men and women—includes DXA and fracture ascertainment (33). The VITAL trial includes marine but not plant-based n-3 PUFAs and is thus not designed to determine skeletal health effects of ALA supplementation. If skeletal outcomes are shown, an examination of mechanism of action via n-3 effects on chronic inflammation is warranted.
Conclusions
This study adds to a body of evidence supporting the association between circulating inflammatory biomarkers as a predictor for incident hip fracture in postmenopausal Caucasian women.
Acknowledgments
We thank Keding Hua and Amy Lehman for their assistance in extracting data from the Women's Health Initiative (WHI).
Authors' Roles in the Study: S.W.I., T.S.O., B.L., R.D.J., study design; B.L., data analysis; S.W.I., T.S.O., B.L., R.D.J., data interpretation; S.W.I., B.L., T.S.O., drafting manuscript; and S.W.I., T.S.O., B.L., M.J.L., K.E.B., J.A.C., R.D.J., review of manuscript.
The findings and conclusions herein are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This trial was registered at clinicaltrials.gov as NCT00000611.
We acknowledge the following WHI investigators: Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, Nancy Geller. Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, Charles Kooperberg, Barbara Cochrane, Julie Hunt, Marian Neuhouser, Lesley Tinker, Susan Heckbert, Alex Reiner. Regional Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson, Kathryn M. Rexrode, Brian Walsh, J. Michael Gaziano, Maria Bueche; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard, Lucile Adams-Campbell, Lawrence Lessin, Cheryl Iglesia, Brian Walitt, Amy Park; (The Ohio State University, Columbus, OH) Rebecca Jackson, Randall Harris, Electra Paskett, W. Jerry Mysiw, Michael Blumenfeld; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick, Mark A. Hlatky, Manisha Desai, Jean Tang, Stacy T. Sims; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson, Tamsen Bassford, Cheryl Ritenbaugh, Zhao Chen, Marcia Ko; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende, Maurizio Trevisan, Ellen Smit, Amy Millen, Michael LaMonte; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher, Michael Perri, Andrew Kaunitz, R. Stan Williams, Yvonne Brinson; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace, James Torner, Susan Johnson, Linda Snetselaar, Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller, Jane Cauley, N. Carole Milas; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson, Suzanne Satterfield, Rongling Li, Stephanie Connelly, Fran Tylavsky; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker, Stephen Rapp, Claudine Legault, Mark Espeland, Laura Coker, Michelle Naughton.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Disclosure Summary: S.W.I. has received honoraria for ad hoc advisory board participation with NPS Pharmaceuticals and Alexion Pharmaceuticals. The other authors have nothing to disclose.
Footnotes
- ALA
- α-LA
- CI
- confidence interval
- DHA
- docosahexaenoic acid
- EPA
- eicosapentaenoic acid
- FFQ
- food frequency questionnaire
- HT
- hormone therapy
- LA
- linoleic acid
- MET
- metabolic equivalent
- n-3
- omega-3
- n-6
- omega-6
- NSAID
- nonsteroidal anti-inflammatory drug
- OR
- odds ratio
- PUFA
- polyunsaturated fatty acid
- RBC
- red blood cell
- TNFα-sR1 and -sR2
- TNFα soluble receptor 1 and 2.
References
- 1. Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68:1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. [DOI] [PubMed] [Google Scholar]
- 3. Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157:1023–1031. [DOI] [PubMed] [Google Scholar]
- 4. Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20:1633–1650. [DOI] [PubMed] [Google Scholar]
- 5. Haentjens P, Magaziner J, Colón-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Int Med. 2010;152:380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nanes MS. Tumor necrosis factor-α: molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15. [DOI] [PubMed] [Google Scholar]
- 7. Johnson RA, Boyce BF, Mundy GR, Roodman GD. Tumors producing human tumor necrosis factor induced hypercalcemia and osteoclastic bone resorption in nude mice. Endocrinology. 1989;124:1424–1427. [DOI] [PubMed] [Google Scholar]
- 8. Kindle L, Rothe L, Kriss M, Osdoby P, Collin-Osdoby P. Human microvascular endothelial cell activation by IL-1 and TNF-α stimulates the adhesion and transendothelial migration of circulating human CD14+ monocytes that develop with RANKL into functional osteoclasts. J Bone Miner Res. 2006;21:193–206. [DOI] [PubMed] [Google Scholar]
- 9. Cauley JA, Danielson ME, Boudreau RM, et al. Inflammatory markers and incident fracture risk in older men and women: the Health Aging and Body Composition Study. J Bone Miner Res. 2007;22:1088–1095. [DOI] [PubMed] [Google Scholar]
- 10. Aderka D, Engelmann H, Shemer-Avni Y, et al. Variation in serum levels of the soluble TNF receptors among healthy individuals. Lymphokine Cytokine Res. 1992;11:157–159. [PubMed] [Google Scholar]
- 11. Aderka D. The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine Growth Factor Rev. 1996;7:231–240. [DOI] [PubMed] [Google Scholar]
- 12. Van Zee KJ, Kohno T, Fischer E, Rock CS, Moldawer LL, Lowry SF. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor α in vitro and in vivo. Proc Natl Acad Sci USA. 1992;89:4845–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barbour KE, Boudreau R, Danielson ME, et al. Inflammatory markers and the risk of hip fracture: the Women's Health Initiative. J Bone Miner Res. 2012;27:1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barbour KE, Lui LY, Ensrud KE, et al. Inflammatory markers and risk of hip fracture in older white women: the Study of Osteoporotic Fractures. J Bone Miner Res. 2014;29:2057–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orchard TS, Cauley JA, Frank GC, et al. Fatty acid consumption and risk of fracture in the Women's Health Initiative. Am J Clin Nutr. 2010;92:1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farina EK, Kiel DP, Roubenoff R, Schaefer EJ, Cupples LA, Tucker KL. Dietary intakes of arachidonic acid and α-linolenic acid are associated with reduced risk of hip fracture in older adults. J Nutr. 2011;141:1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Orchard TS, Ing SW, Lu B, et al. The association of red blood cell n-3 and n-6 fatty acids with bone mineral density and hip fracture risk in the Women's Health Initiative. J Bone Miner Res. 2013;28:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trebble T, Arden NK, Stroud MA, et al. Inhibition of tumour necrosis factor-α and interleukin 6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant co-supplementation. Brit J Nutr. 2003;90:405–412. [DOI] [PubMed] [Google Scholar]
- 19. Zwart SR, Pierson D, Mehta S, Gonda S, Smith SM. Capacity of omega-3 fatty acids or eicosapentaenoic acid to counteract weightlessness-induced bone loss by inhibiting NF-κB activation: from cells to bed rest to astronauts. J Bone Miner Res. 2010;25:1049–1057. [DOI] [PubMed] [Google Scholar]
- 20. Huang JT, Welch JS, Ricote M, et al. Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. [DOI] [PubMed] [Google Scholar]
- 21. Duque G. As a matter of fat: new perspectives on the understanding of age-related bone loss. Int Bone Miner Soc. 2007;4:129–140. [Google Scholar]
- 22. Deckelbaum RJ, Worgall TS, Seo T. n-3 Fatty acids and gene expression. Am J Clin Nutr. 2006;83(6 suppl):1520S—1525S. [DOI] [PubMed] [Google Scholar]
- 23. Haag M, Magada ON, Claassen N, Böhmer LH, Kruger MC. Omega-3 fatty acids modulate ATPases involved in duodenal Ca absorption. Prostaglandins Leukot Essent Fatty Acids. 2003;68:423–429. [DOI] [PubMed] [Google Scholar]
- 24. Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 25. Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–S128. [DOI] [PubMed] [Google Scholar]
- 26. Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–S17. [DOI] [PubMed] [Google Scholar]
- 27. Robbins J, Aragaki AK, Kooperberg C, et al. Factors associated with 5-year risk of hip fracture in postmenopausal women. JAMA. 2007;298:2389–2398. [DOI] [PubMed] [Google Scholar]
- 28. Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gold R, Michael YL, Whitlock EP, et al. Race/ethnicity, socioeconomic status, and lifetime morbidity burden in the Women's Health Initiative: a cross-sectional analysis. J Womens Health (Larchmt). 2006;15:1161–1173. [DOI] [PubMed] [Google Scholar]
- 30. Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. [DOI] [PubMed] [Google Scholar]
- 31. Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. [DOI] [PubMed] [Google Scholar]
- 32. Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–1271. [PubMed] [Google Scholar]
- 33. Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]