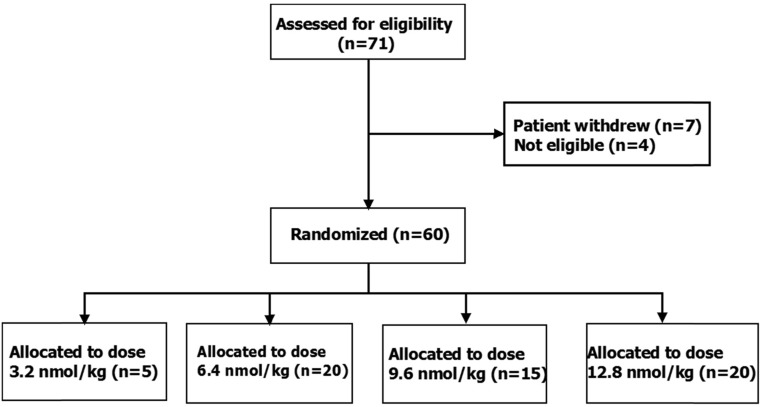
Figure 1.
Patient flow diagram showing the number of patients assessed for eligibility, study enrolment, and kisspeptin-54 dose allocation. The study had a prospective adaptive design whereby the first 15 patients were randomly assigned 1:1:1 to receive kisspeptin-54 at doses of 3.2, 6.4, or 12.8 nmol/kg (n = 5 per group) to trigger oocyte maturation during IVF treatment. After interim analysis of oocyte maturation, subsequent patients were randomly assigned 1:1:1 to receive 6.4, 9.6, or 12.8 nmol/kg (n = 15 per group). No patients were lost to followup and no patients discontinued the intervention.