Abstract
Context:
Seasonal variability in 25-hydroxyvitamin D [25(OH)D] and PTH levels in the general population has been associated with differences in bone turnover markers, bone density, and fracture risk. Seasonal variability in 25(OH)D and PTH levels has also been reported in primary hyperparathyroidism (PHPT).
Objective:
Given the widespread use of vitamin D supplements, we sought to determine whether patients with PHPT still demonstrated seasonal variation in 25(OH)D levels.
Design and Setting:
This cross-sectional study was conducted at a university medical center at a Northeastern U.S. latitude (New York, NY).
Patients:
One hundred patients with PHPT participated in the study.
Outcome Measures:
We assessed vitamin D supplement use and seasonal variation in serum 25(OH)D.
Results:
Patients had PHPT ([mean ± SD] calcium, 10.8 ± 1.0 mg/dL; PTH, 85 ± 48 pg/mL) with a mean 25(OH)D level of 29 ± 10 ng/mL. Although only one fifth of participants had vitamin D deficiency (19% < 20 ng/mL), more than half were either deficient or insufficient (54% < 30 ng/mL). Sun exposure varied by season, but there were no seasonal differences in levels of 25(OH)D, PTH, bone markers, or bone mineral density, or in the prevalence of 25(OH)D less than 20 or less than 30 ng/mL. Most of the participants (65%) took supplemental vitamin D (dose among users: mean, 1643 ± 1496 IU; median, 1000 IU daily), and supplement users had markedly better vitamin D status than nonusers (25(OH)D < 20 ng/mL: 8 vs 40%; P < .0001; < 30 ng/mL: 40 vs 80%; P = .0001; ≥ 30 ng/mL: 60 vs 20%; P = .0001).
Conclusions:
We found no evidence of seasonal variation in 25(OH)D levels or PHPT disease severity in the Northeastern United States. This change is likely due to widespread high vitamin D supplement intake, which has resulted in better vitamin D status among supplement users and can mask the effect of season on serum 25(OH)D levels.
Vitamin D status as measured by 25-hydroxyvitamin D [25(OH)D] levels is affected by both sun exposure and vitamin D intake (1, 2). Seasonal variation in 25(OH)D levels has been demonstrated in multiple population-based studies, both in the young and elderly, across various countries and latitudes, and in institutionalized and free-living populations (3–10). Wintertime nadirs in 25(OH)D levels have been associated with increased PTH levels, increased markers of bone turnover, lower areal bone mineral density (BMD) by dual-energy x-ray absorptiometry (DXA), and higher fracture rates as well as a higher proportion of falls resulting in fracture (4, 8, 10, 11). In a randomized controlled open-label study in Germany, supplementation with calcium and vitamin D in the winter prevented seasonal changes in bone turnover and bone loss (12).
Similar seasonal variability in 25(OH)D levels that inversely correlate with PTH levels has been previously reported in cohorts with primary hyperparathyroidism (PHPT) living at a northeastern latitude in the United States (New York City: 40.67°N, 73.9°W), as well as in Japan (36°N, 138°E) and Denmark (56°N, 10°E) (13–15). Independent of season, vitamin D deficiency has been reported to exacerbate hyperparathyroidism in PHPT, and has been associated with more pronounced biochemical, densitometric, and skeletal microarchitecture abnormalities (16, 17). Given that seasonal changes in 25(OH)D are associated with higher PTH levels in PHPT, such variations could also affect the skeletal disease this disorder.
Recently, increased public awareness of the potential detrimental effects of vitamin D deficiency not only on the skeleton but on other health outcomes as well, has led to increasing vitamin D supplementation. Several large population studies in nonhyperparathyroid cohorts have documented a decline in vitamin D deficiency (18, 19). Although not reported in these studies, if the improvement was due to increased vitamin D supplement use throughout the year, an effect on seasonal changes in 25(OH)D and PTH might be expected.
In 2008, the third International Workshop on Asymptomatic Primary Hyperparathyroidism recommended that 25(OH)D levels be measured in all patients with PHPT, and that patients be supplemented to a minimum 25(OH)D level of 20 ng/mL (20). This was reaffirmed in the recent fourth International Workshop Guidelines (21, 22). Until that time, many physicians did not routinely recommend vitamin D supplements in patients with PHPT, who already had hypercalcemia and often hypercalciuria. However, similar to the general population, recent data demonstrates an increase in vitamin D supplementation in patients with mild PHPT (23). Given these trends in vitamin D supplementation and their effect on the prevalence of vitamin D deficiency and insufficiency, this study sought to determine whether seasonal variability in 25(OH)D levels is evident in patients with mild PHPT in the Northeastern United States. We also investigated whether previously demonstrated seasonal differences in PTH levels, and potential differences in skeletal indices are apparent. Demonstration of seasonal variability in 25(OH)D levels and disease severity in PHPT could support season-specific vitamin D targets or greater vitamin D supplementation in the winter in PHPT, a population already at risk for skeletal loss and fracture.
Materials and Methods
This is a cross-sectional analysis of 25(OH)D levels and vitamin D status using common clinical cut-points for vitamin D deficiency [defined as 25(OH)D level < 20 ng/mL] and vitamin D deficiency or insufficiency [defined as 25(OH)D level < 30 ng/mL] by season of enrollment. This was a secondary analysis of data collected from a study evaluating the effect of vitamin D status on skeletal and mineral metabolism parameters in patients with PHPT including areal bone mineral density (aBMD) by DXA, markers of bone turnover, and calciotropic hormones as well as other indices of mineral metabolism. All patients gave written, informed consent. This study was approved by the Institutional Review Board at the Columbia University Medical Center.
Participants
Potential participants with primary hyperparathyroidism were referred from the ambulatory clinics of Endocrine Surgery, General Endocrinology, and The Metabolic Bone Diseases Unit at the Columbia University College of Physicians and Surgeons, New York Presbyterian Hospital. Participants (N = 100) representing consecutive patients who met eligibility criteria and consented to participate in the study, were enrolled between December 2010 and February 2014. All participants had PHPT, diagnosed by the presence of hypercalcemia (calcium > 10.2 mg/dL) and an elevated or inappropriately normal PTH level. None had thiazide-induced hyperparathyroidism or familial hypocalciuric hypercalcemia (excluded on the basis of family history and 24-h urine calcium excretion). Exclusion criteria included bisphosphonate use within the past 2 years; current use of cinacalcet or denosumab; current or previous use of prednisone 7.5 mg for more than 6 months; current or past use of carbamazepine, phenytoin, or phenobarbital for more than 3 months; malignancy within 5 years other than nonmelanomatous skin cancer; granulomatous diseases; HIV; serum creatinine level at least 1.5 mg/dL; liver disease; gastrointestinal diseases known to affect calcium metabolism or cause secondary hyperparathyroidism such as Crohn's disease, celiac disease or gastric bypass; and pregnancy. Both symptomatic (ie, those with nephrolithiasis) and asymptomatic PHPT and those meeting and not meeting criteria for parathyroidectomy were enrolled.
Clinical and biochemical evaluation
Demographic data, medical history, and medication use were obtained from participants. Fasting samples for serum calcium (normal range, 8.7–10.2 mg/dL), phosphate (normal range, 2.5–4.3 mg/dL), albumin (normal range, 3.5–5.5 g/dL), and creatinine (normal range, 0.5–1.2 mg/dL), as well as urine calcium (normal range, 150–300 mg/24 hours) were measured by an automated chemistry analyzer. PTH was measured by immunoradiometric assay for intact PTH (normal range, 10–66 pg/mL) (Scantibodies Laboratory). Serum 25-hydroxyvitamin D (25(OH)D; normal range, 30–80 ng/mL) and serum 1,25-dihydroxyvitamin D (1,25(OH)2D; normal range, 15–75 pg/mL) were measured by liquid chromatography/tandem mass spectrometry (Quest Diagnostics). Bone-specific alkaline phosphatase (BSAP; normal premenopausal range, 11.5–29.6 U/L; postmenopausal range, 14.2–42.6 U/L) was measured by ELISA (Quidel Corp) and carboxy-terminal telopeptides of type 1 collagen (CTX; normal premenopausal range, 0.112–0.738 ng/mL; postmenopausal range, 0.142–1.351 ng/mL) by ELISA (Immunodiagnostics Systems). Estimated glomerular filtration rate (GFR) (normal range, > 60 mL/min/1.73 m2) was calculated using the Modification of Diet in Renal Disease equation (24).
Calcium and vitamin D intake
Calcium and vitamin D intake was assessed by validated questionnaire, which included questions on food sources containing calcium and vitamin D, such as dairy products and fish, as well as specific questions about multivitamins, calcium, vitamin D, and other supplements (25).
Sun exposure questionnaire
Sun exposure was assessed by validated questionnaire designed to elicit information about the amount of sunlight exposure that a subject received in a typical week (26). Information collected included the amount of time spent in the sunlight in a typical week (daily, > 3 times/wk or < 3 times/wk, each episode lasting < 20 min, between 20 min and 1 h, and > 1 h), whether arms were bare or not, the type of clothing worn (short sleeves, shorts, hats), sunscreen use, recent travel, and season. Baseline scores range from 0 for a homebound person who is never in the sun to 24 for a person who is in the sun for more than 1 hour at least three times a week during the summer months. Additional points were given for travel to lower latitudes in the previous month, based upon location and duration of visit.
Definition of season
Seasons were defined by the summer and winter solstices, and spring and autumn equinoxes in New York over the enrollment period (Spring, March 20 to June 20; Summer, June 21 to September 21; Autumn, September 22 to December 20; Winter, December 21 to March 19) (27).
DXA
aBMD was measured at the lumbar spine, L1–L4; total hip; femoral neck; and one-third radius (Hologic Inc.). In vivo precision, determined according to the standard method at this facility, is 1.28% at the lumbar spine, 1.36% at the hip, and 0.70% for the distal radius (1/3 site) (28).
Statistical analysis
Data are presented as mean ± SD and percentages except where otherwise noted. Between-group differences in demographic, biochemical, and skeletal indices were evaluated by χ2, ANOVA, independent two-sample two-sided t test, Fisher's exact test, or Jonckheere trend test as appropriate. Critical test values were adjusted for unequal variances when appropriate. Seasonal variation in vitamin D levels and other related parameters were assessed with ANOVA. Seasons were also grouped together in different combinations to perform further analyses that reflected the time lag of 25(OH)D production in response to sunlight exposure and that accounted for extremes of sunlight exposure. When two groups were formed, an independent two-sample two-sided t test was performed to assess for between-group differences. When more than two groups were formed, ANOVA was performed. χ2 test was used when comparing seasonal variability in categorical variables. Jonckheere trend test was used to evaluate for trend in vitamin D status across consecutive months and seasons. Analyses were conducted with SAS, Version 9.3 (SAS Institute). Two-sided P < .05 were considered to suggest statistical significance.
Results
Clinical and biochemical data
In this cohort of 100 PHPT patients, participants were predominantly white, non-Hispanic women (Table 1) who had biochemical evidence of PHPT (serum calcium [mean ± SD], 10.8 ± 1.0 mg/dL; PTH, 85 ± 48 pg/mL). The mean 25(OH)D level was 29 ± 10 ng/mL (range, 6–54 ng/mL). Although less than one fifth of participants had vitamin D deficiency (19% < 20 ng/mL), more than half were either deficient or insufficient in vitamin D (54% < 30 ng/mL).
Table 1.
Participant Demographics Overall and by Season
| Characteristic | Overall (n = 100) | Spring (n = 26) | Summer (n = 18) | Autumn (n = 24) | Winter (n = 32) | ANOVA P Value |
|---|---|---|---|---|---|---|
| Age, y, mean ± sd | 62 ± 12 | 62 ± 10 | 59 ± 11 | 66 ± 13 | 60 ± 14 | .24 |
| Female, % | 79 | 85 | 78 | 83 | 72 | .62 |
| Race, % White | 85 | 96 | 83 | 79 | 81 | .27 |
| Ethnicity, % Non-Hispanic | 83 | 85 | 72 | 83 | 84 | .71 |
| BMI, kg/m2, mean ± sd | 28 ± 6 | 27 ± 5 | 26 ± 7 | 29 ± 7 | 29 ± 5 | .17 |
The mean vitamin D intake was 1493 ± 1574 IU daily between diet and supplementary sources. Supplementation with vitamin D was common, with 65% of participants taking a mean and median dose of 1643 ± 1496 IU and 1000 IU per day, respectively. Despite their hypercalcemia, 21 patients were taking vitamin D doses of 2000 IU or more daily, and three subjects were taking prescription ergocalciferol 50 000 IU weekly.
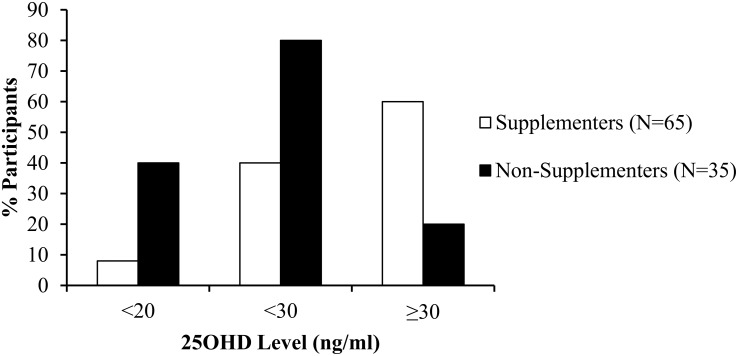
All participants had some dietary vitamin D intake, with a mean intake of 445 ± 476 IU daily. Given that dietary vitamin D intake was comparable between those who did and did not consume supplements (465 ± 505 vs 409 ± 421 IU/d; P = .58), total vitamin D intake was significantly higher in the supplementation subgroup (2077 ± 1658 vs 409 ± 421 IU/d; P < .0001). Compared with the participants not taking supplementation, those on supplementation had higher 25(OH)D levels (33 ± 10 ng/mL vs 22 ± 8 ng/mL; P < .05) and lower prevalence of 25(OH)D < 20 and < 30 ng/mL (8 and 40% vs 40 and 80%; P < .0001 and P = .0001, respectively; Figure 1). Mean sun exposure score among participants was 8.7 (maximum score, 24) and did not differ among those who did and did not consume vitamin D supplements (8.7 ± 5.3 vs 8.8 ± 6.5; P = .91).
Figure 1.
Vitamin D status by supplementation use. Difference by χ2 test between supplementers (white) and nonsupplementers (black) using common 25(OH)D thresholds: <20, <30, and ≥ 30 ng/mL.
Seasonal variation
Patients enrolled in different seasons did not differ in age, sex, race, ethnicity, or body mass index (BMI; Table 1). There were no differences in the serum levels of 25(OH)D, PTH, 1,25(OH)2D, calcium, or in the prevalence of 25(OH)D < 20 and < 30ng/mL in the overall group by season (Table 2). The 35 participants not on vitamin D supplements similarly had no seasonal differences in serum 25(OH)D (ANOVA P = .66), PTH (P = .80), or 1,25(OH) 2D levels (P = .91) or in the prevalence of 25(OH)D < 20 ng/mL (P = .72) or 25(OH)D < 30 ng/mL (P = .65). Nor were seasonal differences in vitamin D status found among the subgroup of 35 lean participants with BMI < 25 kg/m2 (data not shown).
Table 2.
Vitamin D Status and Biochemistries: Overall and by Season
| Measurementa | Overall (n = 100) | Spring (n = 26) | Summer (n = 18) | Autumn (n = 24) | Winter (n = 32) | ANOVA P Value |
|---|---|---|---|---|---|---|
| 25(OH)D < 20 ng/ml | 19 (19) | 4 (15) | 4 (22) | 2 (8) | 9 (28) | .29 |
| 25(OH)D < 30 ng/ml | 54 (54) | 12 (46) | 9 (50) | 13 (54) | 20 (63) | .65 |
| Serum 25(OH)D, ng/mL | 29 ± 10 | 31 ± 11 | 30 ± 12 | 29 ± 6 | 26 ± 11 | .26 |
| Serum PTH, pg/mL | 85 ± 48 | 88 ± 47 | 69 ± 41 | 86 ± 39 | 92 ± 59 | .44 |
| Serum 1,25(OH)2D, pg/mL | 69 ± 24 | 69 ± 25 | 64 ± 22 | 83 ± 36 | 71 ± 26 | .22 |
| Serum calcium, mg/dL | 10.8 ± 1.0 | 10.7 ± 0.6 | 10.7 ± 0.5 | 10.7 ± 0.7 | 10.7 ± 0.7 | .99 |
| GFR, mL/min/1.73 m2 | 84 ± 24 | 88 ± 35 | 80 ± 16 | 81 ± 15 | 85 ± 24 | .74 |
| Serum phosphorous, mg/dL | 3 ± 0.5 | 3 ± 0.5 | 3 ± 0.6 | 3 ± 0.4 | 3 ± 0.4 | .93 |
| Serum albumin, g/dL | 4.5 ± 0.3 | 5 ± 0.3 | 5 ± 0.3 | 4 ± 0.2 | 5 ± 0.3 | .45 |
| Urine calcium, mg/d | 250 ± 144 | 251 ± 127 | 200 ± 137 | 257 ± 136 | 265 ± 163 | .56 |
| CTX, ng/mL | 0.6 ± 0.4 | 0.6 ± 0.3 | 0.6 ± 0.5 | 0.7 ± 0.3 | 0.7 ± 0.4 | .95 |
| BSAP, U/L | 40 ± 19 | 32 ± 15 | 42 ± 3 | 47 ± 18 | 41 ± 12 | .05b |
Data are presented as N (%) or mean ± sd.
Normal ranges: 25(OH)D, 30–80 ng/mL; PTH, 10–66 pg/mL; 1,25(OH) 2D, 15–75 pg/mL; calcium, 8.7–10.2 mg/dL; GFR, > 60 mL/min/1.73 m2; phosphorous, 2.5–4.3 mg/dL; albumin, 3.5–5.5 g/dL; urine calcium, 150–300 mg/d; CTX premenopausal range, 0.112–0.738 ng/mL; CTX postmenopausal range, 0.142–1.351 ng/mL; BSAP premenopausal range, 11.5–29.6 U/L; BSAP postmenopausal range 14.2–42.7 U/L.
P values: spring vs autumn, P = .01; differences among other seasons were not significant.
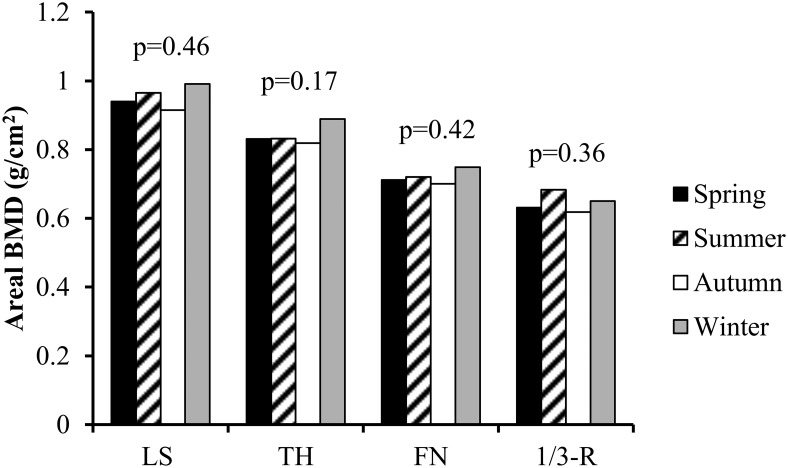
To account for the time-lag of 25(OH)D production in response to sunlight exposure we also analyzed the data by comparing winter and spring combined vs summer and autumn combined (25(OH)D, 28 ± 11 vs 30 ± 9 ng/mL; PTH, 90 ± 53 vs 79 ± 41; 1,25(OH)2D, 70 ± 25 vs 69 ± 23 pg/mL; 25(OH)D < 20 ng/mL, 22 vs 14%; 25(OH)D < 30 ng/mL, 55 vs 52%; P = NS for all), as well as spring vs autumn (25(OH)D, PTH, 1,25(OH)2D, 25(OH)D < 20 and 25(OH)D < 30 ng/mL; P = NS for all; Table 2). Similarly, analyses performed to reflect the extremes of sunlight exposure by comparing winter vs summer did not show any differences in 25(OH)D, PTH, 1,25(OH)2D, or prevalence of 25(OH)D < 20 or < 30 ng/mL (Table 2; P = NS for all). There was also no difference in 25(OH)D, PTH, or 1,25(OH) 2D levels or in prevalence of 25(OH)D < 20 or 25(OH)D < 30 ng/mL when comparing consecutive months in 2-month blocks (Table 3). There were no seasonal differences in biochemical or skeletal indices of PHPT disease severity including the bone turnover marker C-telopeptide of collagen (Table 2) or aBMD at any site (Figure 2). BSAP level was lower in spring vs autumn whereas levels in all other seasons were similar (Table 2).
Table 3.
Vitamin D Status and Biochemistries: Consecutive 2-Month Blocks
| Month | Jan-Feb (n = 24) | Mar-Apr (n = 21) | May-Jun (n = 13) | Jul-Aug (n = 14) | Sept-Oct (n = 11) | Nov-Dec (n = 17) | ANOVA P-Value |
|---|---|---|---|---|---|---|---|
| 25(OH)D < 20 ng/ml | 9 (38) | 4 (19) | 0 (0) | 3 (21) | 1 (9) | 2 (12) | .10 |
| 25(OH)D < 30 ng/ml | 16 (67) | 10 (48) | 7 (54) | 6 (43) | 6 (55) | 9 (53) | .25 |
| Serum 25(OH)D, ng/mL | 24 ± 11 | 31 ± 12 | 30 ± 7 | 31 ± 11 | 32 ± 11 | 28 ± 7 | .15 |
| Serum PTH, pg/mL | 97 ± 10 | 84 ± 11 | 91 ± 13 | 65 ± 13 | 87 ± 15 | 83 ± 33 | .55 |
| Serum 1,25(OH) 2D, pg/mL | 70 ± 5 | 71 ± 5 | 62 ± 7 | 68 ± 7 | 76 ± 7 | 68 ± 6 | .86 |
Data are presented as N (%) or mean ± sd.
Figure 2.
aBMD by season. ANOVA in BMD of lumbar spine (LS), total hip (TH), femoral neck (FN), 1/3 distal radius (1/3R) across the Spring (black), Summer (diagonal stripe), Autumn (white), and Winter (gray).
As expected, sun exposure (total sun score; Table 4) did vary by season of enrollment with the highest exposure in the summer and the lowest in the winter (16 ± 5 vs 5 ± 3; P < .0001), and a similar pattern was seen in those who did not take supplemental vitamin (15 ± 6 vs 5 ± 5; P = .005). There was no difference by season in dietary, supplemental, and total calcium and vitamin D intake or in the percentage of patients taking vitamin D supplementation (Table 4). Among those not consuming supplements, dietary vitamin D intake did not vary by season (P = .51). When the data were analyzed by sex or racial/ethnic subgroups, no seasonal variations were observed in any of the above parameters (data not shown). Omitting the single African American participant did not affect the results (data not shown).
Table 4.
Seasonal Variation in Sun Exposure Score and Daily Calcium and Vitamin D Intake
| Measurement | Spring (n = 26) | Summer (n = 18) | Autumn (n = 24) | Winter (n = 32) | ANOVA P-Value |
|---|---|---|---|---|---|
| Vitamin D supplementation | 15 (58) | 10 (56) | 16 (67) | 24 (75) | .12 |
| Daily supplemental vitamin D intake, IU | 1067 ± 1509 | 1130 ± 1785 | 1391 ± 1689 | 768 ± 800 | .45 |
| Daily dietary vitamin D, IU | 413 ± 398 | 290 ± 203 | 526 ± 732 | 484 ± 386 | .41 |
| Daily total vitamin D intake, IU | 1480 ± 1615 | 1420 ± 1780 | 1917 ± 2001 | 1228 ± 938 | .45 |
| Daily calcium intake, mg/dL | 1260 ± 856 | 931 ± 642 | 868 ± 702 | 1170 ± 862 | .28 |
| Sun exposure score (max = 24) | 8 ± 5 | 16 ± 5 | 9 ± 4 | 5 ± 3 | < .0001 |
Data are presented as N (%) or mean ± sd.
Discussion
In our cohort of patients with PHPT at a Northeastern U.S. latitude, vitamin D intake was high and primarily came from supplemental rather than dietary sources. Approximately two thirds of patients were taking significant amounts of supplemental vitamin D. Not surprisingly, we found the incidence of vitamin D deficiency and insufficiency to be markedly lower in those PHPT patients who were on vitamin D supplements. Although we documented expected seasonal differences in sun exposure, there was no evidence for seasonal variability in 25(OH)D or PTH levels or in BMD. Although we observed minor differences in BSAP levels by season (specifically a lower level in the spring), these variations were small in magnitude, inconsistent across seasons, and unlikely to be of clinical importance. We suspect this lack of seasonal variability in vitamin D levels is due, at least in part, to the high level of vitamin D supplementation, the use and quantity of which did not differ by season.
Studies in the general population in the United States and Canada have also noted a high prevalence of vitamin D supplementation as well as an increase in food fortification with vitamin D (19, 29). This phenomenon has been associated with a concomitant decline in vitamin D deficiency. The Canadian Multicenter Osteoporosis Study found that over a 10-year period, vitamin D supplement intake increased by 317% and 193% in women and men, respectively, accompanied by a corresponding increase in serum 25(OH)D levels and decrease in PTH levels (19). The prevalence of vitamin D deficiency (25(OH)D < 20 ng/mL) and insufficient or deficient levels (25(OH)D < 30 ng/mL) decreased from 30% and 76–20% and 60%, respectively. Similarly, in the Study of Women's Health Across the Nation, the prevalence of vitamin D deficiency declined over 11 years among all racial/ethnic groups, not only among supplement users from 35 to 6%, but also in nonsupplement users from 51 to 39% (29).
The results of this report differ from our findings 20 years ago, in a PHPT cohort from the same geographic location (15). In the previous cohort, marked seasonal differences were noted in serum 25(OH)D levels, with the lowest levels seen in winter months. Furthermore, a linear trend in 25(OH)D levels was demonstrated when analyzed by consecutive 2-month blocks, with the highest 25(OH)D levels in September–October, likely reflecting the lag in vitamin D production after high summer sun exposure. These seasonal changes in 25(OH)D levels were inversely correlated with PTH, and associated with further increases in already elevated PTH levels, although not with changes in aBMD. Notably, none of the participants in the prior cohort took vitamin D supplements and they also tended to avoid dairy products.
Only two other studies have assessed for seasonal variation in 25(OH)D levels in patients with primary PHPT. In a study by Moosgaard et al (14) conducted in Denmark, lower 25(OH)D levels and higher prevalence of vitamin D deficiency were noted in the winter compared with the summer in both PHPT and age-matched controls without PHPT. Lower 25(OH)D levels were associated with higher levels of calcium, PTH, and alkaline phosphatase and lower renal calcium excretion and femoral neck and forearm aBMD, as well as a trend toward increased risk of osteoporotic fractures. In another study, Yamashita et al (13) also noted seasonal variation in 25(OH)D levels in PHPT patients living in Japan, with highest levels in the summer and lowest levels in the winter, but there was no seasonal variation in PTH, 1,25(OH)2D, or other clinical, biochemical, or densitometric indices.
Possible explanations for our discrepant results may include differences in extremes of sunlight exposure, the lower average levels of 25(OH)D, higher prevalence of vitamin D deficiency, and lower rates of supplementation in the other cohorts compared with ours. The Danish population was exposed to wider extremes of sunlight exposure, with only two seasons: summer (defined as April–September, when it is light much of the time at their latitude; median, 25(OH)D 14 ng/mL) and winter (defined as October–March, dark much of the time; median, 25(OH)D 11ng/mL). In the Japanese cohort, mean 25(OH)D level was similarly low (15 ng/mL). Furthermore, only 8% of the Denmark cohort reported taking vitamin D supplements. Although vitamin D intake and supplementation was not reported for the Japanese cohort, we suspect that vitamin D intake was low as other studies have reported low dietary and supplementary intake of vitamin D, as well as lack of routine food fortification with vitamin D in Asian populations (30–33).
However, we found no seasonal variation in 25(OH)D or PTH levels even in the minority of our cohort not consuming supplements despite seasonal variation in sun exposure. This is consistent with the findings of the Study of Women's Health Across the Nation noted above, in which the incidence of vitamin D deficiency also declined in supplement nonusers (11). Fortification of food products may account for some of this shift. In the current cohort, even those participants not taking vitamin D supplementation had an average daily dietary vitamin D intake of 409 ± 421 IU. In addition, we may have been underpowered to see any seasonal variation in the subset of our cohort not taking supplemental vitamin D, in which only 14 subjects (across all seasons) had 25(OH)D in the deficient range. Other factors might have also blunted the effect of sun exposure on seasonal variation in serum 25(OH)D levels. Although our sun exposure questionnaire did account for certain barriers to sunlight such as use of sunscreen, hats, and types of clothing, other factors such as ozone/pollution acting as barriers to reduce absorption of UVB-irradiation in the summertime may have limited our ability to detect differences (34). In addition, we did not measure skin phototype or body surface area exposed to sun by season, which might have improved our sun exposure measurement (35, 36).
This study has several limitations in addition to those regarding adequacy of sun exposure assessment. Because of its cross-sectional design, we cannot infer within-individual seasonal variation in vitamin D levels over time. Our sample size could have limited our ability to detect between-group differences, although with the 100 subjects, we had 80% power to detect a 9.6-ng/dL difference in 25(OH)D levels across the four seasons (one-way ANOVA, F-test, P = .05). Our study also has several important strengths. Our cohort is relatively large for a study of PHPT and is representative of patients with PHPT seen in the United States today. We assessed many factors that might influence seasonal variability in 25(OH)D levels, including a measure of sun exposure as well as dietary and supplemental vitamin D and BMI. Few studies have addressed the effect of season on 25(OH)D levels in patients with PHPT, and none in patients taking the recommended dietary or supplemental vitamin D.
In summary, patients with PHPT in the New York City metropolitan area are following secular trends and increasing their vitamin D supplement use. Most were taking over-the-counter vitamins, and it is clearly advisable for physicians who care for PHPT patients to specifically query regarding supplement use. The seasonal variability in 25(OH)D levels or in markers of PHPT disease severity that we documented 20 years ago in a cohort with PHPT from the same geographic location no longer exists. The findings suggest that PHPT disease severity is not exacerbated in the winter months in populations with high levels of vitamin D supplementation. This study represents the first assessment of seasonal 25(OH)D variation in a PHPT cohort with high vitamin D intake. We conclude that the lack of seasonal variability in 25(OH)D levels in a Northeastern U.S. cohort with PHPT is due, at least in part, to high vitamin D supplement intake, which can mask the effect of season on vitamin D.
Acknowledgments
Author Contributions: E.C., acquisition of data, data analysis and interpretation, drafting of manuscript. M.D.W., acquisition of data, data analysis and interpretation, drafting of manuscript. A.K., acquisition of data, manuscript revision. C.Z., data analysis, manuscript revision. D.J.M., data analysis, manuscript revision. S.J.S., design, acquisition of data, interpretation of data, manuscript drafting and revision.
This work was supported by National Institutes of Health Grants R01 DK084986 and K24 DK074457 as well as the Joseph Weintraub Family Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 25OHD
- 25-hydroxyvitamin D
- aBMD
- areal bone mineral density
- BMI
- body mass index
- BMD
- bone mineral density
- BSAP
- bone-specific alkaline phosphatase
- CTX
- carboxy-terminal telopeptides of type 1 collagen
- DXA
- dual-energy x-ray absorptiometry
- GFR
- glomerular filtration rate
- PHPT
- primary hyperparathyroidism.
References
- 1. Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–378. [DOI] [PubMed] [Google Scholar]
- 2. Dawson-Hughes B, Dallal GE, Krall EA, Harris S, Sokoll LJ, Falconer G. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann Intern Med. 1991;115(7):505–512. [DOI] [PubMed] [Google Scholar]
- 3. Rosecrans R, Dohnal JC. Seasonal vitamin D changes and the impact on health risk assessment. Clin Biochem. 2014;47(7–8):670–672. [DOI] [PubMed] [Google Scholar]
- 4. Darling AL, Hart KH, Gibbs MA, Gossiel F, Kantermann T, Horton K, et al. Greater seasonal cycling of 25-hydroxyvitamin D is associated with increased parathyroid hormone and bone resorption. Osteoporos Int. 2014;25(3):933–41. [DOI] [PubMed] [Google Scholar]
- 5. van Schoor NM, Knol DL, Deeg DJ, Peters FP, Heijboer AC, Lips P. Longitudinal changes and seasonal variations in serum 25-hydroxyvitamin D levels in different age groups: Results of the Longitudinal Aging Study Amsterdam. Osteoporos Int. 2014;25(5):1483–1491. [DOI] [PubMed] [Google Scholar]
- 6. McKinney K, Breitkopf CR, Berenson AB. Association of race, body fat and season with vitamin D status among young women: A cross-sectional study. Clin Endocrinol. 2008;69(4):535–541. [DOI] [PubMed] [Google Scholar]
- 7. Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–777. [DOI] [PubMed] [Google Scholar]
- 8. Melin A, Wilske J, Ringertz H, Sääf M. Seasonal variations in serum levels of 25-hydroxyvitamin D and parathyroid hormone but no detectable change in femoral neck bone density in an older population with regular outdoor exposure. J Am Geriatr Soc. 2001;49(9):1190–1196. [DOI] [PubMed] [Google Scholar]
- 9. Pérez-Llamas F, López-Contreras MJ, Blanco MJ, López-Azorín F, Zamora S, Moreiras O. Seemingly paradoxical seasonal influences on vitamin D status in nursing-home elderly people from a Mediterranean area. Nutrition. 2008;24(5):414–420. [DOI] [PubMed] [Google Scholar]
- 10. Pasco JA, Henry MJ, Kotowicz MA, et al. Seasonal periodicity of serum vitamin D and parathyroid hormone, bone resorption, and fractures: The Geelong Osteoporosis Study. J Bone Miner Res. 2004;19(5):752–758. [DOI] [PubMed] [Google Scholar]
- 11. Cauley JA, Greendale G, Ruppert K, et al. Serum 25 Hydroxyvitamin D (25(OH)D), Bone Mineral Density (BMD) and Fracture Risk across the Menopausal Transition: Study of Women's Across the National (SWAN). J Bone Miner Res. 2014;29(Suppl 1). [Google Scholar]
- 12. Meier C, Woitge HW, Witte K, Lemmer B, Seibel MJ. Supplementation with oral vitamin D3 and calcium during winter prevents seasonal bone loss: A randomized controlled open-label prospective trial. J Bone Miner Res. 2004;19(8):1221–30. [DOI] [PubMed] [Google Scholar]
- 13. Yamashita H, Noguchi S, Uchino S, Watanabe S, Koike E, Murakami T, et al. Vitamin D status in Japanese patients with hyperparathyroidism: Seasonal changes and effect on clinical presentation. World J Surg. 2002;26(8):937–41. [DOI] [PubMed] [Google Scholar]
- 14. Moosgaard B, Vestergaard P, Heickendorff L, Melsen F, Christiansen P, Mosekilde L. Vitamin D status, seasonal variations, parathyroid adenoma weight and bone mineral density in primary hyperparathyroidism. Clin Endocrinol. 2005;63(5):506–513. [DOI] [PubMed] [Google Scholar]
- 15. Silverberg SJ, Brown I, Bilezikian JP. Seasonal Variation in 25-Hydroxyvitamin D in Primary Hyperparathyroidism. J Bone Miner Res. 1999;14(Suppl 1). [Google Scholar]
- 16. Stein EM, Dempster DW, Udesky J, et al. Vitamin D deficiency influences histomorphometric features of bone in primary hyperparathyroidism. Bone. 2011;48(3):557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silverberg SJ, Shane E, Dempster DW, Bilezikian JP. The effects of vitamin D insufficiency in patients with primary hyperparathyroidism. Am J Med. 1999;107(6):561–567. [DOI] [PubMed] [Google Scholar]
- 18. Bailey RL, Fulgoni VL, 3rd, Keast DR, Dwyer JT. Examination of vitamin intakes among US adults by dietary supplement use. J Acad Nutr Diet. 2012;112(5):657–663e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berger C, Greene-Finestone LS, Langsetmo L, et al. Temporal trends and determinants of longitudinal change in 25-hydroxyvitamin D and parathyroid hormone levels. J Bone Miner Res. 2012;27(6):1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silverberg SJ, Lewiecki EM, Mosekilde L, Peacock M, Rubin MR. Presentation of asymptomatic primary hyperparathyroidism: Proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94(2):351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silverberg SJ, Clarke BL, Peacock M, et al. Current issues in the presentation of asymptomatic primary hyperparathyroidism: Proceedings of the fourth international workshop. J Clin Endocrinol Metab. 2014;99(10):3580–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marcocci C, Bollerslev J, Khan AA, Shoback DM. Medical management of primary hyperparathyroidism: Proceedings of the fourth international workshop on the management of asymptomatic primary hyperparathyroidism. J Clin Endocrinol Metab. 2014;99(10):3607–3618. [DOI] [PubMed] [Google Scholar]
- 23. Walker MD, Cong E, Lee JA, et al. Low vitamin D levels have become less common in primary hyperparathyroidism. Osteoporos Int. DOI:10.1007/500198-015-3199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 25. Hertzler A, Frary R. A dietary rapid assessment method (RAM). Top Clin Nutr. 1994;9:76–85. [Google Scholar]
- 26. Stein EM, Strain G, Sinha N, et al. Vitamin D insufficiency prior to bariatric surgery: Risk factors and a pilot treatment study. Clin Endocrinol (Oxf). 2009;71(2):176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. The Old Farmer's Almanac 2013: Yankee Publishing Incorporated; 2012. [Google Scholar]
- 28. Bonnick SL, Johnston CC, Jr, Kleerekoper M, et al. Importance of precision in bone density measurements. J Clin Densitom. 2001;4(2):105–110. [DOI] [PubMed] [Google Scholar]
- 29. Mitchell D, Lee H, Greendale G, Cauley J, et al. Increasing 25-hydroxyvitamin D levels over time: The Study of Women's Health Across the Nation (SWAN). J Bone Miner Res. 2014;29(Suppl 1):S1.25712350 [Google Scholar]
- 30. Ashwell M, Stone EM, Stolte H, et al. UK Food Standards Agency Workshop Report: An investigation of the relative contributions of diet and sunlight to vitamin D status. Br J Nutr. 2010;104(4):603–611. [DOI] [PubMed] [Google Scholar]
- 31. Macdonald HM, Mavroeidi A, Fraser WD, et al. Sunlight and dietary contributions to the seasonal vitamin D status of cohorts of healthy postmenopausal women living at northerly latitudes: A major cause for concern? Osteoporos Int. 2011;22(9):2461–2472. [DOI] [PubMed] [Google Scholar]
- 32. Lips P. Vitamin D status and nutrition in Europe and Asia. J Steroid Biochem Mol Biol. 2007;103(3–5):620–625. [DOI] [PubMed] [Google Scholar]
- 33. Lips P. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol. 2010;121(1–2):297–300. [DOI] [PubMed] [Google Scholar]
- 34. Lips P, van Schoor NM, de Jongh RT. Diet, sun, and lifestyle as determinants of vitamin D status. Ann N Y Acad Sci. 2014;1317:92–98. [DOI] [PubMed] [Google Scholar]
- 35. Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessing Fitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149(11):1289–1294. [DOI] [PubMed] [Google Scholar]
- 36. Barger-Lux MJ, Heaney RP. Effects of above average summer sun exposure on serum 25-hydroxyvitamin D and calcium absorption. J Clin Endocrinol Metab. 2002;87(11):4952–4956. [DOI] [PubMed] [Google Scholar]