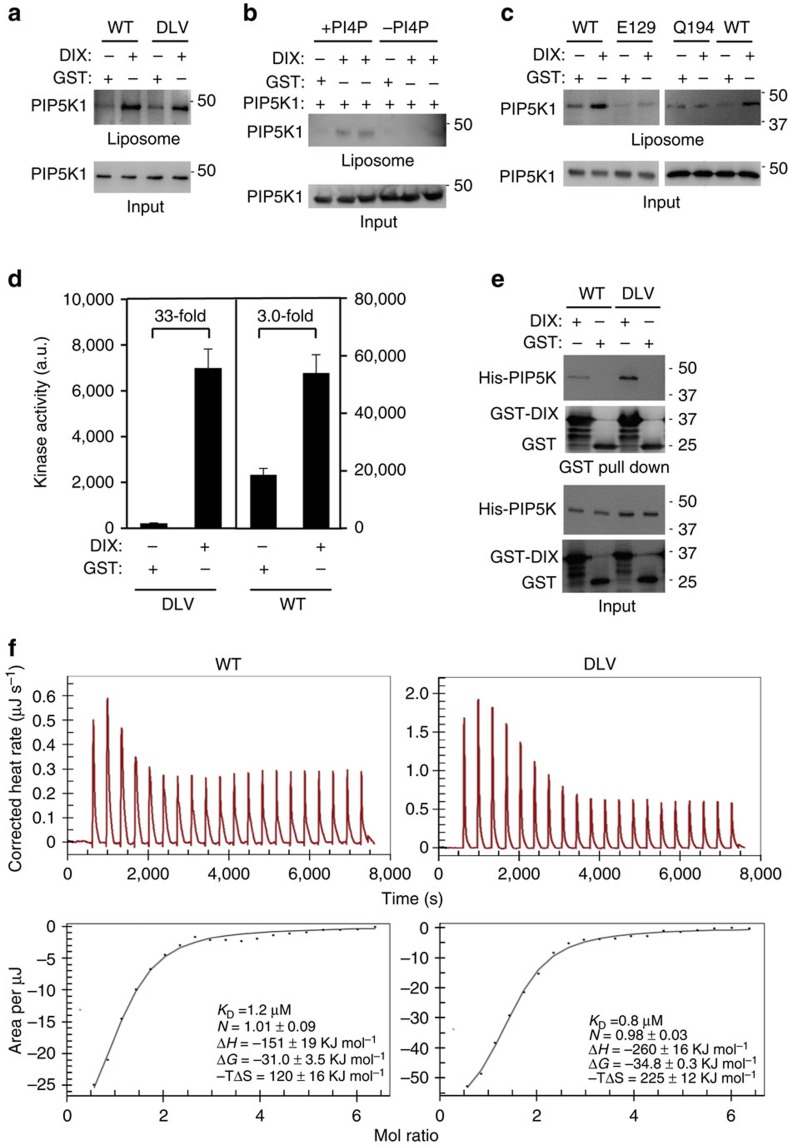
Figure 5. Regulation of zPIP5K1A enzymatic activity.
(a) Binding of zPIP5K1A (wild type (WT)) and its monomeric DLV mutant to PtdIns4P-containing liposomes measured by using a liposome floatation assay in the presence of DIX or GST. (b) DIX stimulates PIP5K1 binding only to liposomes containing PtdIns4P. The liposome floatation assay was performed using phosphatidylcholine/phosphatidylserine liposomes with or without PtdIns4P. (c) Effect of the E129A and Q194A mutations on Dvl-induced binding of zPIP5K1A to PtdIns4P-containing liposomes. (d) Effects of DIX on the kinase activity of zPIP5K1A (WT) and its monomeric DLV mutant. Data are presented as means±s.d. (e) Interaction of zPIP5K1A (WT) and its monomeric DLV mutant with DIX. GST-pull-down was performed with His-tagged zPIP5K1A proteins and GST-tagged DIX with GST as a control. The proteins were detected by western blotting. (f) Assessment of the binding affinities of zPIP5K1A (WT) and its monomeric DLV mutant for DIX using ITC. The experiments were repeated twice times. The results from one of the experiments are shown.