Figure 2.
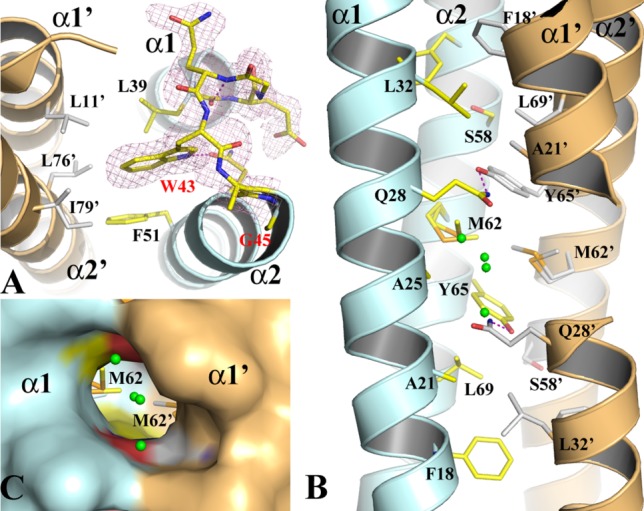
The structure of the WT-EsxB AP dimer. (A) The structure of the inter-helix link drawn in stick format to show the conformation of the conserved WXG motif from monomer A of the AP dimer. The 2FoFc electron density map drawn in magenta mesh is contoured at 1σ. (B) The packing of the core of the helical bundle of the AP dimer. Monomers A and B are in pale cyan and light orange, respectively. Key residues contributing to the interaction across the dimer interface are drawn in stick format. Primes on labels for residues or secondary structures refer to those from monomer B. A pair of hydrogen bonds between Q28 and Y67’ are drawn in magenta dash line. Water molecules are drawn in small green spheres. (C) A molecular surface representation of part of the three-way tunnel within the core of the helical bundle of the AP dimer. Two other smaller entrances to the tunnel are on the two sides between α1 and α2, α1’ and α2’, respectively.
