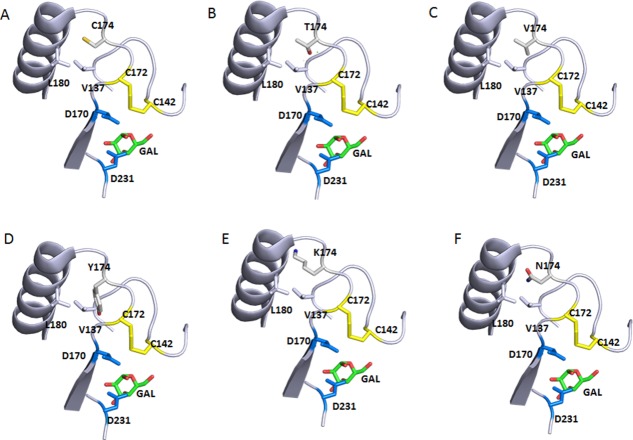
Figure 9.
Local environment of Cys174 and the effect of selective mutations. Only part of the backbone are shown for simplifications. The atom coloring scheme is same as in Figure 1 except for what is specified for each panel. Catalytic residues D170 and D231 and bound product GAL are shown too. The neighboring C142/C172 disulfide is also shown. Structure is from 3HG5.4 (A) WT Cys174 sulfhydryl sulfur in yellow. (B) C174T mutation with the Threonine hydroxyl oxygen in red. (C) C174V. (D) C174Y with the Tyrosine hydroxyl oxygen in red. C174Y would not fit in this position. (E) C174K with the Lysine amine nitrogen in blue. C174K clashes with the neighboring α-helix. (F) C174N with the amide oxygen in red, and nitrogen in blue. C174N can be potentially glycosylated as it introduces a consensus glycosylation NXS/T sequence and clashes with the neighboring α-helix.