Figure 1.
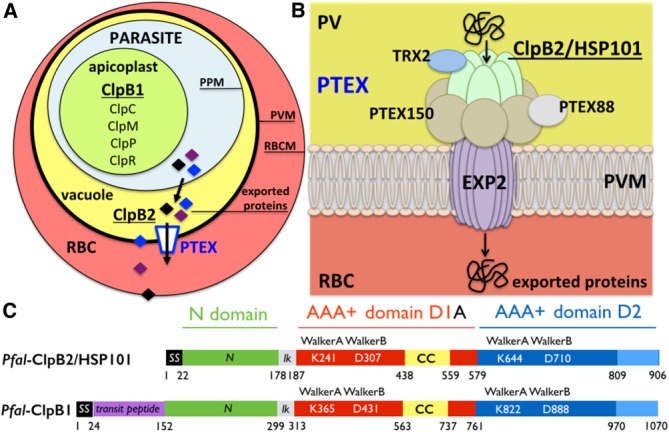
The Clp proteases and chaperones in Plasmodium and the Plasmodium translocon of exported proteins. (A) Simplified diagram showing a red blood cell (RBC) infected by a parasite encased in the parasitophorous vacuole. The nomenclature and localization of all Plasmodium Clp chaperones and proteases are show. There are two ClpB proteins, ClpB1 and ClpB2/HSP101. All Clp proteins are localized in the apicoplast except the vacuolar ClpB2/HSP101. (B) ClpB2/HSP101 is a subunit of the parasite-derived Plasmodium translocon of exported proteins (PTEX). Plasmodium proteins are translocated across the parasitophorous vacuole membrane (PVM) into the red blood cell cytoplasm through a translocation pore composed of Exported protein-2 (EXP2). Prior to their transport, exported proteins are unfolded by the hexameric AAA+ protein ClpB2/HSP101. For some cargos, unfolding also necessitates reduction of disulfide bonds by the accessory subunit thioredoxin-2 (TRX2). The other subunits, PTEX150 and PTEX88, have unknown functions. The arrows indicate the directionality of transport from the parasitophorous vacuole (yellow) towards the erythrocyte cytoplasm (red). The relative positions of the different subunits within the complex are unknown. (C) Domain organization of the two ClpB proteins present in Plasmodium falciparum: the apicoplastic ClpB1 and the vacuolar ClpB2/HSP101. The signal sequence (SS in black) targets proteins to the general secretory pathway and the transit peptide (in magenta) is a specific signal that specifies subcellular localization to the apicoplast. The N-terminal domain (in green) is linked to the two catalytic nucleotide-binding domains (NBD) D1 (in red) and D2 (in blue) by a short basic linker (lk in grey). The D1 domains of ClpB and ClpC chaperones are characterized by the presence of a coiled-coil insertion, the middle domain also named “arm” (CC in yellow). The D2 domains usually contain a C-terminal module (in light blue). Each NBD contains two signature Walker (A and B) motifs.
