Abstract
Bendamustine is approved in the United States for relapsed indolent lymphoma. However, it has not been widely studied in mantle cell lymphoma (MCL). We retrospectively reviewed the records of all patients with MCL who were treated with bendamustine at three centers. The primary endpoint was overall response rate (ORR). Thirty patients with MCL received bendamustine, 25 for relapsed disease. After a median follow-up of 12 months, there were 15 complete responses (CRs) with an ORR of 83% (95% confidence interval [CI] 70–97%). Factors significantly associated with longer survival were achieving a CR and classical (versus blastic) variant of MCL. Grade 3 or 4 neutropenia, anemia and thrombocytopenia occurred in 23%, 3% and 20%, respectively. There was one case of progressive multifocal leukoencephalopathy 10 months after therapy completion. Bendamustine in combination with rituximab demonstrated a high response rate in this study of patients with predominantly relapsed MCL.
Keywords: Non-Hodgkin lymphoma, mantle cell lymphoma, chemotherapy, bendamustine, rituximab
Introduction
In 2011, an estimated 66 000 cases of non-Hodgkin lymphoma (NHL) will be diagnosed in the United States [1], with 6–8% of these cases being mantle cell lymphoma (MCL) [2]. The hallmark of MCL is a translocation between chromosomes 11 and 14, resulting in cyclin D1 overexpression [3]. The natural history of MCL is heterogeneous, with some patients following an indolent course, but in most cases the disease behaves aggressively [4], with over 90% presenting with extranodal disease [5]. As the understanding of this disease has evolved from an indolent to a more aggressive paradigm, the application of more intensive chemotherapy regimens including anthracyclines and high-dose cytarabine has led to improved survival rates in recent years [6].
MCL is generally chemotherapy sensitive, but the induced responses are often short-lived [7]. The usual frontline treatment approach in the USA consists of intensive chemoimmunotherapy [8–10]. The role of transplant in the treatment of MCL, as frontline therapy as well as for relapsed disease, remains controversial, with some investigators showing benefit for autologous hematopoietic stem cell transplant (HSCT) [9] and others for allogeneic HSCT [11].
Bendamustine, which was initially developed in the 1960s in Eastern Europe, has recently evolved as an increasingly used chemotherapeutic agent for diverse lymphomas in the United States and Europe [12]. Bendamustine had been used for decades in East Germany in the treatment of chronic lymphocytic leukemia (CLL), Hodgkin lymphoma disease, non-Hodgkin lymphoma (NHL), multiple myeloma and lung cancer. It is an alkylating agent with a different mechanism of action compared to other alkylating agents [13]. In 2008 the US Food and Drug Administration (FDA) approved its use as a first-line agent for CLL and for relapsed indolent B-cell NHL [14–16].
There have been many studies testing bendamustine in the treatment of indolent lymphoma, but none have focused exclusively on MCL. Kahl et al. used single-agent bendamustine and reported a 75% overall response rate (ORR) and a 14% complete response (CR) rate in 100 patients with indolent B-cell lymphoma, primarily follicular type [15]. In a German study, 63 patients with indolent lymphoma (including 16 with MCL) received rituximab plus bendamustine [17]. The MCL group had a progression-free survival (PFS) of 10 months. In one of the initial German trials of bendamustine, Herold et al. compared bendamustine, vincristine and prednisone (BOP) to cyclophosphamide, vincristine and prednisone (COP) in 162 patients with indolent lymphomas [18]. Twenty-one of the patients in this study treated with BOP had MCL; in these patients the 5-year survival rate was 43%. Another combination regimen of bendamustine, mitoxantrone and rituximab was studied in 57 patients [19]. Among the 18 patients with MCL, there was an ORR of 78% and a median PFS of 21 months.
Several studies performed in the USA led to bendamustine approval by the FDA [15,16,20]. Friedberg et al. used single-agent bendamustine in the treatment of 76 patients with rituximab-refractory indolent and transformed NHL, yielding an ORR of 77% and a CR rate of 15%. Robinson et al. studied rituximab plus bendamustine for relapsed indolent lymphomas. Of the 66 patients in the study, 11 of the 12 patients with MCL responded to therapy. Recently, other investigators have reported similarly high response rates with bendamustine-containing regimens in small cohorts of patients with MCL [21,22]. Herein we present a multi-institutional series of patients with MCL treated with bendamustine to add to the body of evidence for activity of this agent in MCL.
Patients and methods
We searched our institutions’ institutional review board (IRB)-approved lymphoma databases to retrospectively identify all patients with a diagnosis of MCL at Jackson Memorial Hospital and the Sylvester Comprehensive Cancer Center (both in Miami, FL) and the Stanford Cancer Center (in Stanford, CA). We cross-referenced this search with our chemotherapy records to identify patients who had received bendamustine as part of their treatment regimen for MCL since its approval in 2008 until June 2011. The diagnostic specimens of all included patients were reviewed to confirm the immunophenotype, cyclin D1 expression, cytologic variant and lymphoma growth pattern. Patients whose specimens did not fulfill the World Health Organization (WHO) criteria for MCL, without clear overexpression of cyclin D1 or the presence of t(11;14), were excluded [2]. When possible, immunostaining with an antibody to Ki-67 (Dako, Carpinteria, CA) was performed and quantified in the diagnostic specimens.
We collected clinical information, including baseline performance status, serum lactate dehydrogenase (LDH), Ann Arbor stage, extranodal sites of disease, histological subtype of MCL, Ki-67 expression, β2-microglobulin, peripheral blood lymphocyte count and percentage, and all chemotherapy treatments that the patient had received. The records were also examined for any adverse events that might have been associated with bendamustine and were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [23].
The primary endpoint was ORR, defined using the revised International Working Group criteria for response assessment [24]. Secondary endpoints were overall survival (OS), time to treatment failure (TTF) and safety. Survival analysis was performed using the Kaplan–Meier method [25], with significance level for separation of survival curves assessed using the log-rank test. For the analysis of survival according to response to treatment, the landmark method was employed [26].
Results
We identified 30 patients who met the search criteria, with 17 patients treated at the University of Miami, two at Jackson Memorial Hospital and 11 at Stanford Cancer Center. The median age at diagnosis was 58 years and 77% of the patients were male. The most common extranodal site of involvement at diagnosis was the bone marrow (57%), followed by the gastrointestinal (GI) tract (33%). Most patients presented with advanced disease, with 28% and 69% of the patients having stage III and IV disease at diagnosis, respectively. Seven patients (23%) were diagnosed with the blastic variant of MCL. The majority of patients were in the intermediate (55%) or low (41%) MCL International Prognostic Index (MIPI) risk groups at the time of their initial diagnosis. Of the 25 patients who did not receive bendamustine as front-line therapy, the most widely used regimens were R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; 36%) and R-MACLO-IVAM (rituximab, methotrexate, doxorubicin, cyclophosphamide, vincristine, ifosfamide, etoposide, cytarabine; 28%). Patients had been treated with a median of one chemotherapy regimen prior to receiving bendamustine (range 1–5). Three patients received rituximab as maintenance. Table I summarizes the baseline characteristics of all the patients.
Table I.
Baseline patient characteristics at diagnosis, n = 30.
| n (%) | |
|---|---|
| Male | 23 (77) |
| Age (median) | 58 (range 39–86) |
| ECOG Performance Status | |
| 0 | 17 (63) |
| 1 | 10 (37) |
| Extranodal site | |
| GI tract | 10 (33)* |
| Bone marrow | 17 (57) |
| Ann Arbor stage | |
| III | 8 (28) |
| IVA | 11 (38) |
| IVB | 9 (31) |
| Histological subtype | |
| Blastic | 7 (23) |
| Classical | 23 (77) |
| Ki-67 (for 18 cases) | |
| <10% | 1 (6) |
| 10–30% | 6 (33) |
| >30% | 11 (61) |
| β2-Microgloblulin (for 13 cases) (mg/L) | |
| <3 | 4 (31) |
| >3 | 9 (69) |
| LDH exceeding upper limit of normal | 10 (42) |
| MIPI score (for 22 cases) | |
| Low | 9 (41) |
| Intermediate | 12 (55) |
| High | 1 (5) |
| Frontline therapy prior to bendamustine | |
| R-CHOP | 9 (36) |
| R-MACLO-IVAM | 7 (28) |
| Other† | 9 (36) |
| Number of treatments prior to bendamustine |
|
| One | 15 (60) |
| Two | 8 (32) |
May be an underestimate, as not all patients underwent full endoscopic evaluation as part of their initial diagnostic work-up.
Other regimens include: single-agent rituximab, R-CVP, R-CHOP + Hyper-CVAD, R-Velcade-CVAD, Revlimid, R-CHOP + R-DHAP, and Hyper-CVAD alternating with Rituxan, high-dose ara-C and methotrexate.
ECOG, Eastern Cooperative Oncology Group; GI, gastrointestinal; LDH, lactate dehydrogenase; MIPI, Mantle Cell Lymphoma International Prognostic Index; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, predni sone; R-MACLO-IVAM, rituximab, methotrexate, doxorubicin, cyclophospha mide, vincristine, ifosfamide, etoposide, cytarabine; R-CVP, rituximab, cyclophosphamide, vincristine, prednisone; Hyper-CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone; R-DHAP, rituximab, dexamethasone, ara-C, cisplatin.
Among the 30 patients studied, 28 had received rituximab with bendamustine, and two had received bendamustine monotherapy. Five patients had received bendamustine with rituximab as initial therapy and the remaining 25 (including two patients who received bendamustine monotherapy) for relapsed disease. The patients who had received bendamustine with rituximab as front-line therapy were not considered to be candidates for more aggressive, multiagent chemo-therapy due to advanced age or comorbidities. Due to the retrospective nature of this study, the dose of bendamustine varied according to the physician’s preference. The most common schedule administered was rituximab 375 mg/m2 on day one and bendamustine 100 mg/m2 on days one and two of a 28-day cycle, given for a median of six cycles. Daily doses of bendamustine given in prior studies ranged from 90 mg/m2 [20] to 120 mg/m2 [19]. The daily doses of bendamustine used in this study were 100 mg/m2 ( n = 12), 90 mg/m2 ( n = 11) and 80 mg/m2 ( n = 7). The most common reason for usage of bendamustine at the dose of 80 mg/m2 was the presence of renal failure with reduced creatinine clearance. At the start of therapy, the goal was to deliver six cycles. Nineteen patients received six cycles and 11 patients received fewer than six, for reasons including death related to progression of disease ( n = 5), progressive disease while on therapy after an initial partial response ( n = 2), adverse effects of therapy ( n = 1; grade 4 pancytopenia), CR before completion of six cycles ( n = 1) and treatment ongoing at the time of publication of this study ( n = 1).
Of the 30 patients who received bendamustine, 15 had a CR and 10 had a partial response (PR), for an ORR of 83% (95% confidence interval [CI] 70–97%). Of the five patients who received bendamustine for initial therapy, three achieved a CR and the other two had a PR. The response to treatment is summarized in Table II. Of note, one patient who achieved a PR with bendamustine actually had a radiological CR by positron emission tomography (PET) and computed tomography (CT), but was found to have a microscopic involvement of his bone marrow, and therefore was defined as a PR.
Table II.
Response to therapy.
| n (%) | |
|---|---|
| Received bendamustine as frontline therapy (n = 5) | |
| CR | 3 (60) |
| PR | 2 (40) |
| Received bendamustine for relapsed disease (n = 25) | |
| CR | 12 (48) |
| PR | 8 (32) |
| SD | 3 (12) |
| PD | 2 (8) |
| All patients (n = 30) | |
| CR | 15 (50) |
| PR | 10 (33) |
| SD | 3 (10) |
| PD | 2 (7) |
| Overall response rate | 83% (95% CI 70–97) |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; CI, confidence interval.
Twenty-five patients received bendamustine for relapsed disease, with 15 as second line and 10 as third line or beyond. Of these 25 patients, 12 (48%) had stage IV and eight (32%) had stage III disease at the time of treatment with bendamustine. Among these 25 patients, the median disease-free interval after frontline treatment was 29.8 months (95% CI 26.2–33.4 months). Frontline treatment yielded an ORR of 88% in these 25 patients. Five of these patients had relapsed MCL after an autologous HSCT. Twenty-one of the 23 (91%) patients with the classical variant responded to bendamustine, whereas only four out of seven (57%) with the blastic variant responded ( p = 0.068, two-sided Fisher’s exact test). At the time of this report, five of the seven patients with the blastic subtype have died due to lymphoma.
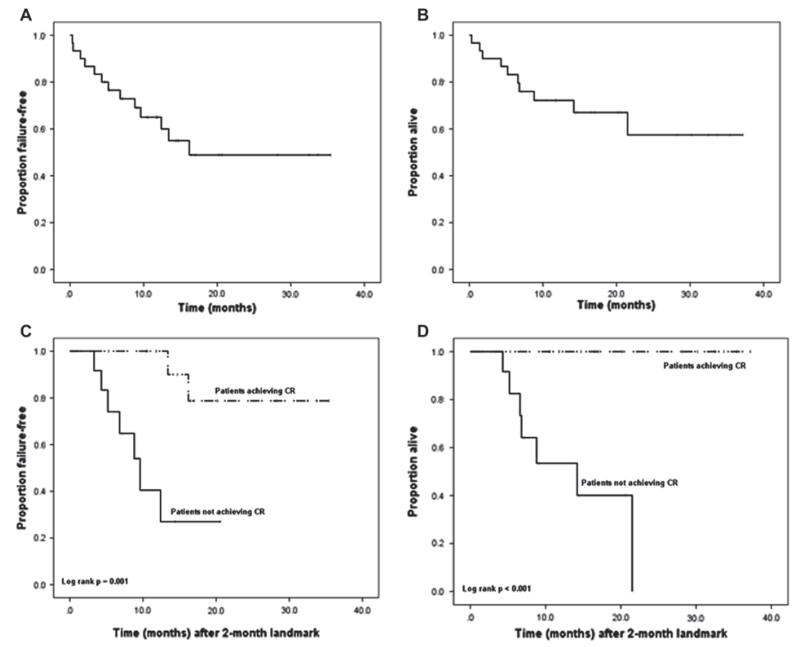
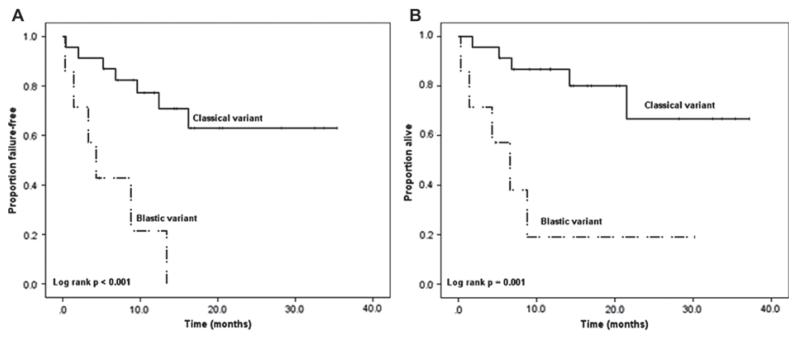
With a median follow-up of 12 months (range 0.3–37.2 months), the median OS was not reached; the 1-year OS rate was 72%; the 2-year OS rate was 57%. When OS was stratified by response (using a 2-month landmark as the starting point for analysis), patients achieving a CR had a significantly longer survival compared to patients who did not achieve a CR (log-rank p < 0.001). The median TTF was 16.2 months. When TTF was stratified by response (again using a 2-month landmark), a statistically significant difference between the two groups was also found; patients who achieved a CR had a significantly longer TTF than patients who did not achieve a CR (log-rank p = 0.001). Figure 1 shows the Kaplan–Meier survival curves for TTF and OS stratified by response. The TTF and OS were significantly worse for patients with the blastic variant versus the classical variant, as shown in Figure 2.
Figure 1.
Kaplan–Meier survival curves showing time to treatment failure in all patients (1A) and stratified by complete response (1C, landmark method); and overall survival in all patients (1B) and stratified by complete response (1D, landmark method).
Figure 2.
Kaplan–Meier survival curves showing time to treatment failure (2A) and overall survival (2B) stratified by blastic versus classical variant of mantle cell lymphoma.
The majority of serious adverse events associated with bendamustine use were hematological (Table III). Grade 3 or 4 neutropenia, anemia, thrombocytopenia and neutropenic fever occurred in 23%, 3%, 20% and 7% of patients, respectively. One patient discontinued therapy with bendamustine after two cycles due to grade IV thrombocytopenia and neutropenia. Of note, blood counts were checked monthly on this study. Two patients required hospitalization for infections (folliculitis and pneumonia) while receiving treatment. Common non-hematologic grade 1 or 2 toxicities were fatigue (27%), edema (20%), erythematous rash (20%) and nausea (13%). One patient had a reactivation of dermatomal herpes zoster during therapy. Another patient was found to have new-onset pulmonary nodules approximately 1 month after completing his course of R-bendamustine for relapsed MCL. His symptoms progressed, and he underwent an open lung biopsy, which revealed CD5-negative, cyclin-D1 negative diffuse large B-cell lymphoma (DLBCL), while biopsy at the time of relapse was CD5-positive, cyclin D1-positive MCL without evidence of DLBCL. He expired shortly thereafter.
Table III.
Adverse events during bendamustine treatment (n = 146 cycles).
| Grade 3–4, n (%) | |
|---|---|
| Hematological | |
| Neutropenia | 7 (23) |
| Neutropenic fever | 2 (7) |
| Anemia | 1 (3) |
| Thrombocytopenia | 6 (20) |
| Leukopenia | 5 (17) |
| Lymphopenia | 6 (20) |
| Non-hematological | |
| Fatigue | 1 (3) |
| Infection | 3 (10) |
| Fever | 1 (3) |
| Near-syncope | 1 (3) |
| Headache | 1 (3) |
| Acute renal failure | 1 (3) |
| Edema | 0 |
| Erythematous rash | 0 |
| Dyspnea | 0 |
| Night sweats | 0 |
| Cough | 0 |
| Pain | 0 |
| Blurred vision | 0 |
| Insomnia | 0 |
| Pruritus | 0 |
| Nausea | 0 |
| Anorexia | 0 |
| Diarrhea | 0 |
| Constipation | 0 |
| Hypocalcaemia | 0 |
| Hypokalemia | 0 |
Two patients developed neurological sequelae during or after treatment with bendamustine. The first patient received six cycles of rituximab plus bendamustine on second relapse and achieved a CR. However, 10 months after completing therapy she began to develop left-sided weakness and dysmetria, along with an ataxic gait. A magnetic resonance imaging (MRI) scan of the brain showed multiple predominantly peripherally enhancing lesions. A stereotactic brain biopsy revealed progressive multifocal leukoencephalopathy (PML) positive for JC virus. At the time of PML diagnosis, she had a low absolute CD4 count of 176 cells/μL (reference range 492–1740), a low absolute CD3 count of 522 (reference range 840–3060) and a normal absolute CD8 count of 368. After 1 year of follow-up, she remains without evidence of relapsed MCL and her neurologic symptoms have gradually improved without therapy, but did not recover completely to baseline. A second patient developed ataxia and incontinence while on bendamustine therapy. Her symptoms continued to progress and she presented to an outside facility 2 months after her last dose of rituximab plus bendamustine. Diagnostic work-up at the time included an unremarkable MRI scan of the brain and spinal cord and negative cerebrospinal fluid cytology. This patient’s symptoms were suspicious for PML, but she died before a definitive diagnosis could be established, and autopsy was declined.
Discussion
Bendamustine has been studied for the treatment of indolent lymphoma both as monotherapy [27] and as part of combination therapy, including combination with rituximab, in an attempt to increase the response rate and survival [16,18,20]. These studies have established bendamustine as a valid option for the treatment of indolent lymphoma. However, despite being grouped together with the indolent lymphomas, MCL often behaves more aggressively, and specific studies in patients with MCL are required.
In the present retrospective study, all five patients who received bendamustine in combination with rituximab as frontline therapy responded. The median TTF in this subset was not reached. Based on these limited data for effectiveness, along with the good tolerability of this regimen, rituximab plus bendamustine may be an option for frontline therapy in patients with MCL who are not candidates for more aggressive treatment, but further study is needed.
In relapsed MCL, we observed an ORR of 80% for bendamustine, similar to responses reported in several distinct frontline regimens in patients with MCL [8,10]. Although the median follow-up in relapsed patients is relatively short (12 months) with a median TTF of 13.4 months, at least four patients remained in continued remission for over 2 years after receiving bendamustine. Furthermore, our results are consistent with the previously reported response rates in small cohorts (7–18 patients) of relapsed MCL treated with bendamustine-based regimens (Table IV).
Table IV.
Summary of studies examining treatment of relapsed mantle cell lymphoma.
| Year | Author | Experimental treatment |
n | ORR | Median TTF (months) |
Median OS (months) |
|---|---|---|---|---|---|---|
| 2010 | Ohmachi | Bendamustine | 11 | 100% | NR | NA |
| 2005 | Rummel | B-R | 16* | 75% | 18 (PFS) | NR |
| 2008 | Robinson | B-R | 12 | 92% | 19.0 (DOR) | NA |
| 2011 | Warsch† | B-R | 25 | 80% | 13.4 | NR |
| 2007 | Weide | B-R-mitoxantrone | 18 | 78% | 21.0 (PFS) | 31 |
| 2011 | Friedberg | B-R-bortezomib | 7 | 71% | NA | NA |
| 2001 | Cohen | FC | 30‡ | 63% | 4.8 (FFS) | 17.5 |
| 2004 | Forstpointer | R-FCM | 52 | 52% | 8.0 (PFS) | NR |
| 2005 | Witzig | Temsirolimus | 35 | 38% | 6.5 (TTP) | 12 |
| 2009 | Hess | Temsirolimus | 54§ | 22% | 4.8 (PFS) | 12.8 |
| 2009 | Habermann | Lenalidomide | 15 | 53% | 5.6 (PFS) | NA |
| 2008 | O’Connor | Bortezomib | 40 | 47% | 5.3 (PFS) | NA |
| 2009 | Goy | Bortezomib | 141 | 40% | 6.7 (TTP) | 23.5 |
Seven of the 16 patients in this study had refractory MCL.
Present study.
Twenty of the 30 patients in this study had relapsed/refractory MCL.
Outcomes shown for the 54 patients allocated to 175/75 mg dosing schedule.
MCL, mantle cell lymphoma; B-R, bendamustine, rituximab; FC, fludarabine, cyclophosphamide; R-FCM, rituximab, fludarabine, cyclophosphamide, mitoxantrone; ORR, overall response rate; TTF, time to treatment failure; NR, not reached; PFS, progression-free survival; DOR, duration of response; NA, data not available; FFS, failure-free survival; TTP, time to progression; OS, overall survival.
Due to the low prevalence of MCL, there have been few studies specifically evaluating non-transplant treatments in the setting of relapsed and refractory disease. Currently, the proteasome inhibitor bortezomib is the only FDA-approved agent for relapsed MCL, based on its single-agent activity for relapsed and refractory MCL within the PINNACLE trial [28]. In this study of 141 patients, 40% of patients responded to treatment and 8% had a CR. The median OS was 23.5 months and time to progression was 6.7 months, with a median duration of response of 9.2 months [29]. A similar study, with a total of 40 patients, compared relapsed to refractory MCL in their response to bortezomib. The ORR and CR of 47% and 12% were similar to the rates found in the PINNACLE trial [30]. Like bendamustine, bortezomib has a toxicity profile that is favorable when compared to the more intensive regimens used to treat MCL.
Lenalidomide was studied in 15 patients with relapsed or refractory MCL. Responses were seen in 53% of the patients, with a 20% CR. The median PFS was 5.6 months [31]. Fludarabine-based chemotherapy and temsirolimus have also been tested in patients with primary and relapsed MCL, with some evidence of activity [32–35].
Although our data are retrospective, the observed response in our cohort of patients seems to be at least similar to, and may be even better than, the responses reported with bortezomib, lenalidomide and other new drugs, as demonstrated in Table IV. Further prospective studies of bendamustine in patients with relapsed MCL as well as side by side comparisons with alternative currently approved therapies will establish its role in these patients.
While our study included only five patients who received bendamustine after autologous HSCT, bendamustine induced a CR in three and a PR in one other. A fifth patient did not tolerate bendamustine. As of this publication, two of the three patients who had a CR from bendamustine have relapsed. Based on these data, bendamustine appears to demonstrate some activity in patients who experience progression of MCL after transplant. In contrast, our data suggest that bendamustine is ineffective in the blastic variant of MCL, and other novel strategies should be tested in this subtype.
The hematological toxicities that we encountered were expected and manageable. They included neutropenia (23%) and thrombocytopenia (20%). One patient withdrew from treatment as a result of these toxicities. There was one confirmed case of PML, which has not previously been described with bendamustine, but this patient was also treated with rituximab, which is associated with PML [36]. This patient currently has no evidence of MCL and is slowly improving from her PML. Another patient with neurological complaints had a clinical suspicion for PML, but expired before biopsy was performed to confirm or rule out this diagnosis. Further reports will clarify whether indeed bendamustine treatment is associated with PML. Despite this concern, compared to other chemotherapy regimens with comparable efficacy in treating patients with MCL, the toxicity profile of bendamustine appears to be favorable. We did not observe an excess of infections during or after therapy as has been reported with the purine analogs such as fludarabine.
Based on the results of this study, bendamustine appears to have a favorable profile as a second-line agent for patients with relapsed MCL. The response rate is at least comparable to that with other therapies, especially when examined in light of the toxicity profile seen with other regimens. This study supports the need for continued investigation of bendamustine as a therapy for patients with MCL in both the frontline and relapsed settings in prospective clinical trials, some of which are currently ongoing.
Acknowledgements
This work was supported by the National Institutes of Health (CA109335 and CA122105), the Fidelity Foundation and the Dwoskin Family Foundation grants to I.S.L.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- [1].Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- [2].Swerdlow SH, Campo E, Harris NL. WHO classification of tumours of haematopoietic and lymphoid tissues. International Agency for Research on Cancer; Lyon, France: 2008. [Google Scholar]
- [3].Fernandez V, Hartmann E, Ott G, et al. Pathogenesis of mantle-cell lymphoma: all oncogenic roads lead to dysregulation of cell cycle and DNA damage response pathways. J Clin Oncol. 2005;23:6364–6369. doi: 10.1200/JCO.2005.05.019. [DOI] [PubMed] [Google Scholar]
- [4].Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27:1209–1213. doi: 10.1200/JCO.2008.19.6121. [DOI] [PubMed] [Google Scholar]
- [5].Dreyling M, Hiddemann W, European MCL Network Current treatment standards and emerging strategies in mantle cell lymphoma. Hematology Am Soc Hematol Educ Program. 2009:542–551. doi: 10.1182/asheducation-2009.1.542. [DOI] [PubMed] [Google Scholar]
- [6].Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27:511–518. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- [7].Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23:1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- [8].Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23:7013–7023. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- [9].Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lossos IS, Hosein PJ, Morgensztern D, et al. High rate and prolonged duration of complete remissions induced by rituximab, methotrexate, doxorubicin, cyclophosphamide, vincristine, ifosfamide, etoposide, cytarabine, and thalidomide (R-MACLO-IVAM-T), a modification of the National Cancer Institute 89-C-41 regimen, in patients with newly diagnosed mantle cell lymphoma. Leuk Lymphoma. 2010;51:406–414. doi: 10.3109/10428190903518345. [DOI] [PubMed] [Google Scholar]
- [11].Tam CS, Bassett R, Ledesma C, et al. Mature results of the M. D. Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphoma. Blood. 2009;113:4144–4152. doi: 10.1182/blood-2008-10-184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Leoni LM. Bendamustine: rescue of an effective antineoplastic agent from the mid-twentieth century. Semin Hematol. 2011;48(Suppl. 1):S4–S11. doi: 10.1053/j.seminhematol.2011.03.002. [DOI] [PubMed] [Google Scholar]
- [13].Garnock-Jones KP. Bendamustine: a review of its use in the management of indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. Drugs. 2010;70:1703–1718. doi: 10.2165/11205860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [14].Tageja N, Nagi J. Bendamustine: something old, something new. Cancer Chemother Pharmacol. 2010;66:413–423. doi: 10.1007/s00280-010-1317-x. [DOI] [PubMed] [Google Scholar]
- [15].Kahl BS, Bartlett NL, Leonard JP, et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study. Cancer. 2010;116:106–114. doi: 10.1002/cncr.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Friedberg JW, Cohen P, Chen L, et al. Bendamustine in patients with rituximab-refractory indolent and transformed non-Hodgkin’s lymphoma: results from a phase II multicenter, single-agent study. J Clin Oncol. 2008;26:204–210. doi: 10.1200/JCO.2007.12.5070. [DOI] [PubMed] [Google Scholar]
- [17].Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:3383–3389. doi: 10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- [18].Herold M, Schulze A, Niederwieser D, et al. Bendamustine, vincristine and prednisone (BOP) versus cyclophosphamide, vincristine and prednisone (COP) in advanced indolent non-Hodgkin’s lymphoma and mantle cell lymphoma: results of a randomised phase III trial (OSHO# 19) J Cancer Res Clin Oncol. 2006;132:105–112. doi: 10.1007/s00432-005-0023-2. [DOI] [PubMed] [Google Scholar]
- [19].Weide R, Hess G, Koppler H, et al. High anti-lymphoma activity of bendamustine/mitoxantrone/rituximab in rituximab pretreated relapsed or refractory indolent lymphomas and mantle cell lymphomas. A multicenter phase II study of the German Low Grade Lymphoma Study Group (GLSG) Leuk Lymphoma. 2007;48:1299–1306. doi: 10.1080/10428190701361828. [DOI] [PubMed] [Google Scholar]
- [20].Robinson KS, Williams ME, van der Jagt RH, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:4473–4479. doi: 10.1200/JCO.2008.17.0001. [DOI] [PubMed] [Google Scholar]
- [21].Friedberg JW, Vose JM, Kelly JL, et al. The combination of bendamustine, bortezomib, and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood. 2011;117:2807–2812. doi: 10.1182/blood-2010-11-314708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ohmachi K, Ando K, Ogura M, et al. Multicenter phase II study of bendamustine for relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci. 2010;101:2059–2064. doi: 10.1111/j.1349-7006.2010.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Common Terminology Criteria for Adverse Events version 4.0 (CTCAE) National Cancer Institute; 2009. Available from: http://www.ctep.cancer.gov/reporting/ctc_v30.html. [Google Scholar]
- [24].Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- [25].Collett D. Modeling survival data in medical research. Chapman & Hall; London, England: 1994. [Google Scholar]
- [26].Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- [27].Heider A, Niederle N. Efficacy and toxicity of bendamustine in patients with relapsed low-grade non-Hodgkin’s lymphomas. Anticancer Drugs. 2001;12:725–729. doi: 10.1097/00001813-200110000-00003. [DOI] [PubMed] [Google Scholar]
- [28].Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- [29].Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20:520–525. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].O’Connor OA, Moskowitz C, Portlock C, et al. Patients with chemotherapy-refractory mantle cell lymphoma experience high response rates and identical progression-free survivals compared with patients with relapsed disease following treatment with single agent bortezomib: results of a multicentre phase 2 clinical trial. Br J Haematol. 2009;145:34–39. doi: 10.1111/j.1365-2141.2008.07466.x. [DOI] [PubMed] [Google Scholar]
- [31].Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145:344–349. doi: 10.1111/j.1365-2141.2009.07626.x. [DOI] [PubMed] [Google Scholar]
- [32].Cohen BJ, Moskowitz C, Straus D, et al. Cyclophosphamide/fludarabine (CF) is active in the treatment of mantle cell lymphoma. Leuk Lymphoma. 2001;42:1015–1022. doi: 10.3109/10428190109097721. [DOI] [PubMed] [Google Scholar]
- [33].Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:3064–3071. doi: 10.1182/blood-2004-04-1323. [DOI] [PubMed] [Google Scholar]
- [34].Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- [35].Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- [36].Carson KR, Bennett CL. Rituximab and progressive multi-focal leukoencephalopathy: the jury is deliberating. Leuk Lymphoma. 2009;50:323–324. doi: 10.1080/10428190902779257. [DOI] [PubMed] [Google Scholar]