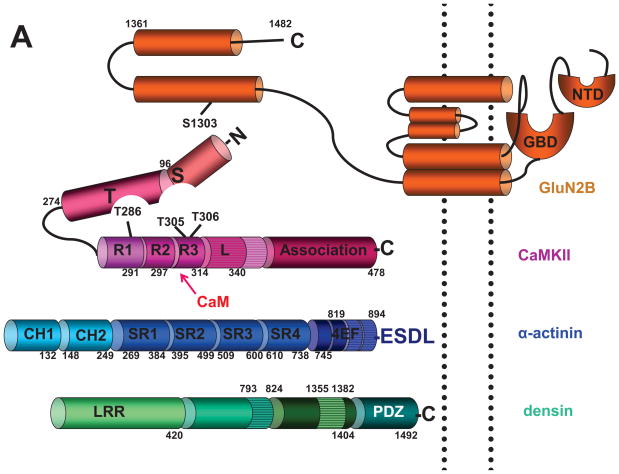
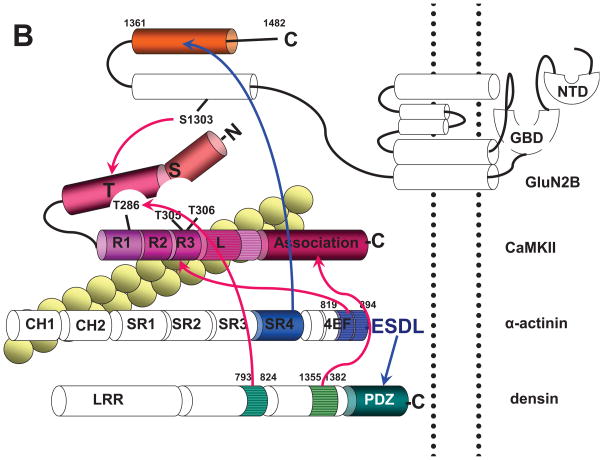
Figure 2. Domains that Mediate Interactions between CaMKII,α-actinin, F-actin, Densin, and NMDARs.
(A) Linear structures of CaMKII and its most prevalent and functionally important binding proteins in spines. The CaMKII diagram (red) shows the N domain (aa 1–96; numbering according to mammalian CaMKIIα), C domain (aa 97–274), catalytic site nested between N and C domains (includes substrate binding site, S site), autoinhibitory segment (aa 275–340) consisting of R1 (aa 275–291; contains T286 for interact with the T site under resting conditions), R2 (aa 291–297; binds to the S site under resting conditions), and R3 (aa 297–314; includes the Ca2+/CaM binding site, which overlaps with R2; dark red oval; T305/T306 inhibit Ca2+/CaM binding if phosphorylated), linker segment L (aa 314–340), and association domain (aa 341–478).
α-Actinin (blue) consists of two calponin homology domains (CH1: aa 1–132; CH2: aa 148–249; numbering according to mammalian α-Actinin-1), four spectrin repeat domains (SR1: aa 269–384; SR2: aa 395–499; SR3: aa 509–600; SR4: 610–738) and four EF hands (aa 745–894). The linker between CH2 and SR1 (aa 250–268) is an important attachment site for the EF hands in the antiparallel dimer (second protomer is not depicted).
Densin (green) is formed by a leucine-rich repeat domain (LRR; consists of 16 leucine-rich repeats; aa 1–420), a central domain of less certain structural identity (aa 421–1404) and a C-terminal PDZ domain (aa 1405–1492).
The NMDAR GluN2B subunit (orange) consists of an extracellular N-terminal domain (NTD) and glutamate binding domain (GBD), which is formed by the N-terminus and the extracellular loop between transmembrane segments 2 and 3, three transmembrane segments, which form, together with a membrane-reentry loop, the pore, and an intracellular C-terminus (aa 838–1482).
(B) Depiction of CaMKII interaction sites. The linker of CaMKIIβ (horizontal stripes), which is of variable length and different from the linker of CaMKIIα, is important for binding to F-actin (yellow beaded double string crossing underneath the linker). The exact binding site on CaMKIIβ for F-actin is unclear. F-actin also binds to CH1 of α-actinin (crossing underneath CH1). Other connections of CaMKII are depicted by red arrows. EF3 and EF4 hands of α-actinin (aa 819–194; horizontal stripes) and Ca2+/CaM compete for binding to R2/R3 on CaMKII (dark red oval). The association domain of CaMKII (aa 341–478) binds to aa 1335–1382 of densin (horizontal stripes) independent of CaMKII activation. The T site of CaMKII binds to aa 1290–1309 of GluN2B (including S1303, which is involved in CaMKII binding and phosphorylated by CaMKII) and aa 793–824 of densin (horizontal stripes) upon addition of Ca2+/CaM or phosphorylation of T286.
α-actinin binds with its C-terminal ESDL motif to the PDZ domain of densin and with its C-terminal portion of SR4 to the C-terminal portion of GluN2B (blue arrows).