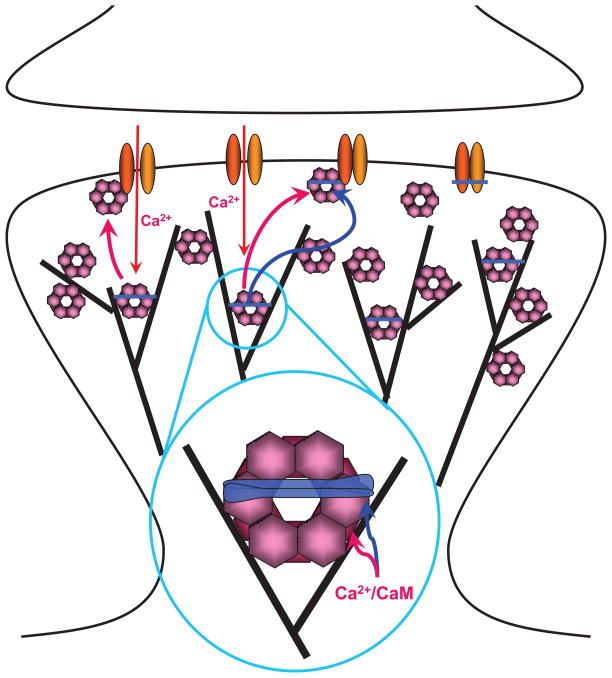
Figure 3. Interactions of CaMKII with F-actin, α -actinin, and NMDARs in Spines.
Under basal conditions CaMKII (pink) is mostly associated with F-actin (black lines). This interaction might also localize CaMKII to an area 50–100 nm underneath the center of the PSD with high CaMKII concentrations also present at the lateral edges of the PSD (Ding et al., 2013). The figure envisions that this association with F-actin occurs in conjunction with α-actinin (blue) as CaMKIIβ subunits within the dodecameric CaMKII complexes as well as α-actinin directly bind to F-actin and to each other. The resulting ‘triades’ (magnified area) are predicted to foster a highly branched F-actin cytoskeleton rather than the parallel fiber arrangement induced by α-actinin alone in the absence of CaMKII.
Ca2+ influx via NMDARs, which consist of GluN1 (yellow) and GluN2 subunits (orange), triggers release of CaMKII from F-actin as Ca2+/CaM will displace CaMKIIβ from F-actin (red arrow in insert) and CaMKIIα and β from α-actinin (blue arrow in insert). CaMKII will then bind to the NMDAR subunit GluN2B (top left area of PSD), which requires either Ca2+/CaM or the more lasting T286/287 autophosphorylation. After removal of Ca2+/CaM, α-actinin will re-associate with CaMKII possibly forming a trimeric complex with GluN2B (top middle area; right area depicts an α-actinin – NMDAR complex without CaMKII).