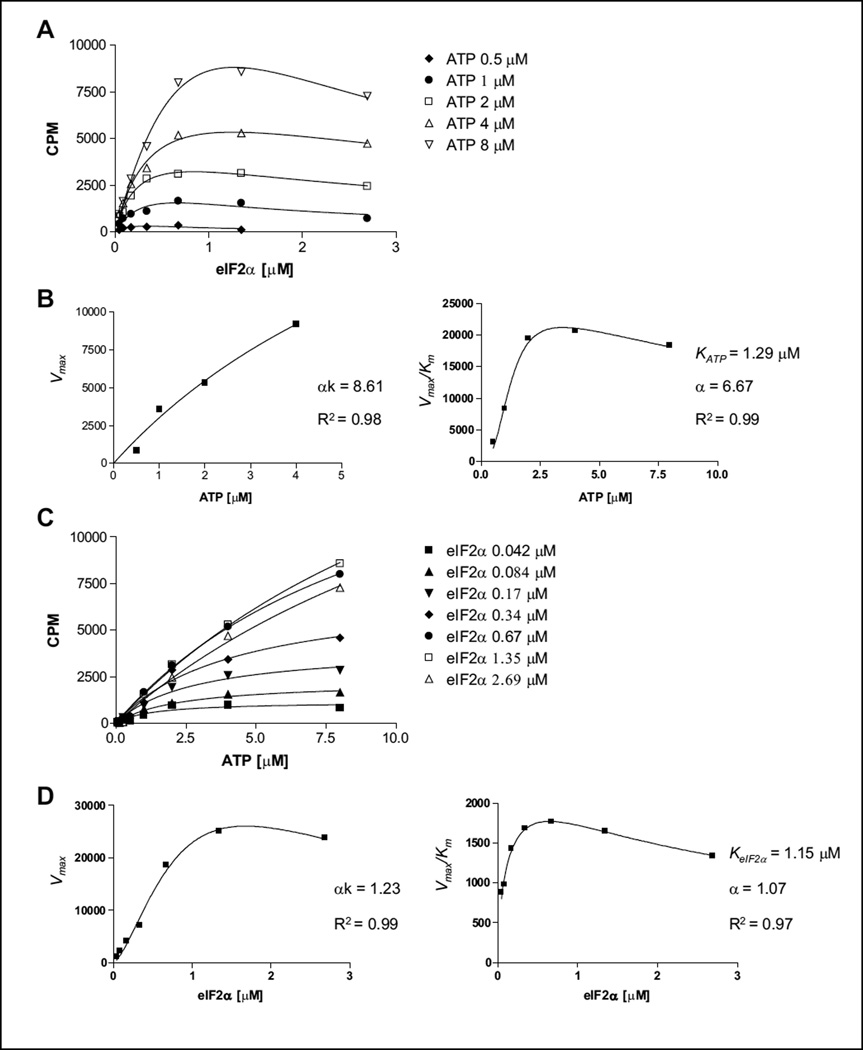
Figure 2.
Kinetic mechnism studies for PERK toward adenosine triphosphate (ATP) and eIF2α substrates. (A) Titration of ATP in the range of 0.5 to 8 µM versus an eIF2a concentration of 0.04 to 3 µM. From each ATP concentration plot, Vmax values of each reaction were calculated. (B) Determination of αk = 8.61 and α = 6.67 values. Based on the curve fit, we demonstrate that PERK kinase follows a random mechanism toward the ATP substrate. (C) Titration of eIF2α in the range of 0.04 to 3 µM versus an ATP concentration of 0.5 to 8 µM. From each eIF2α concentration plot, Vmax values of each reaction were calculated. (D) Determination of αk = 1.23 and α = 1.07 values. Based on the curve fit, we demonstrate that PERK kinase follows a random or steady-state ordered mechanism toward the eIF2α substrate. Experiments were repeated a minimum of three times. One representative experiment is shown.