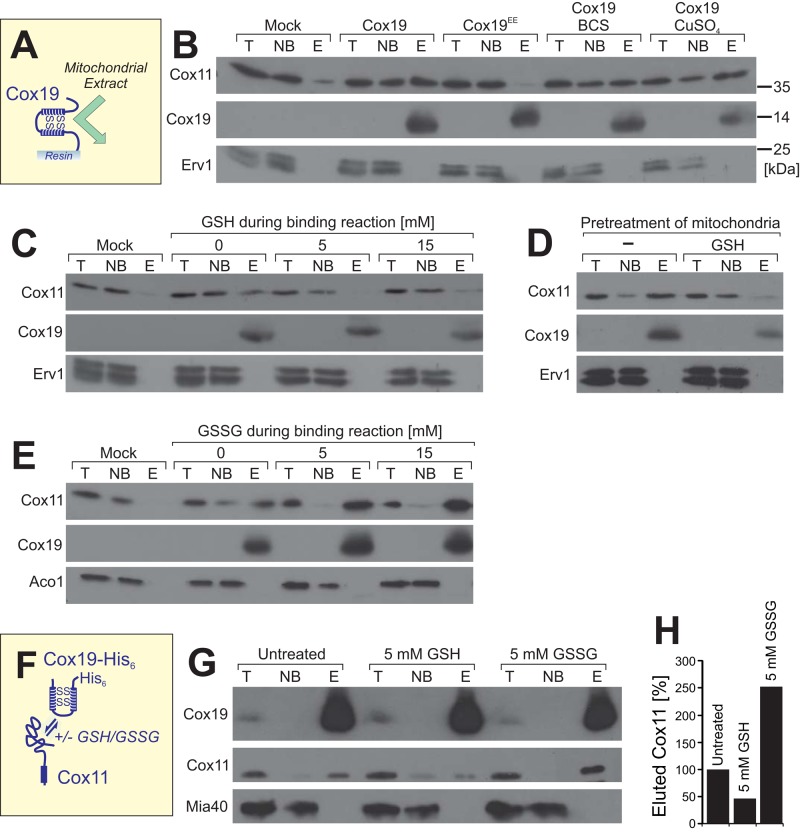
FIGURE 7:
Cox11 binds to Cox19 in a glutathione-dependent manner. (A, B) Purified Cox19-His6 or Cox19EE-His6 was immobilized on Sepharose beads and incubated with extracts of mitochondria expressing Cox11-HA. After washing, proteins of the total (T), not-bound (NB), and bound (E, eluate) fractions were analyzed by Western blotting. Cox11 was detected by use of an HA antibody. For the mock sample, naive Sepharose beads were used that carried neither Cox19-His6 nor Cox19EE-His6. In the samples labeled with BCS and CuSO4, 4 mM bathocuproine disulfonate or 100 μM copper sulfate, respectively, was added to 450 μg of wild-type mitochondria. After 15 min of incubation, the mitochondria were recovered by centrifugation and lysed. The clarified lysate was incubated with Cox19-coated Sepharose beads. (C,E) Pull-down experiments with naive beads (mock) or immobilized Cox19-His6 (other samples) as described in B, with the exception that different amounts of reduced glutathione (GSH) or oxidized glutathione disulfide (GSSG) were present during the binding reaction. (D) Mitochondria were preincubated with 5 mM reduced glutathione. The glutathione was removed by washing. Mitochondria were lysed, and the resulting extract was used for binding experiments as described in B. (F–H) Mitochondria expressing Cox19 with a hexahistidine tag were pretreated in the absence or presence of 5 mM GSH or GSSG for 15 min at 30°C. The mitochondria were washed and lysed in a buffer containing 1% Triton X-100, and the extract was clarified by centrifugation. Cox19-His6 was precipitated by 1 h incubation with Ni-NTA Sepharose and, after several washing steps, eluted with 250 mM imidazole. The T and NB samples correspond to 1% of the material used for the E sample. The samples were analyzed by Western blotting, and the signal intensities were quantified.