FIGURE 9:
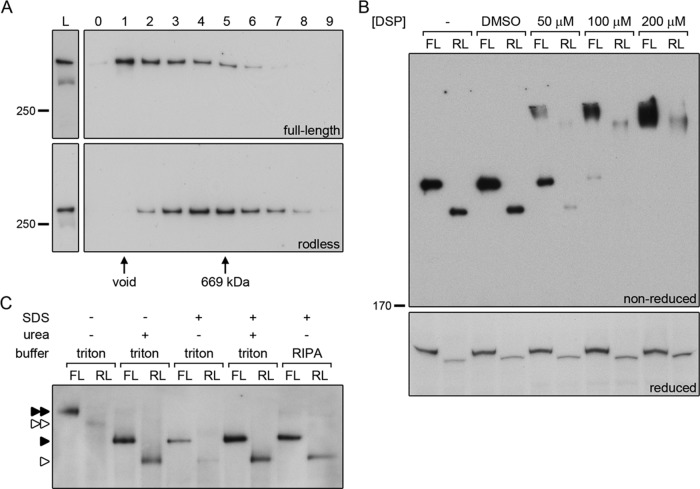
The rod domain is not essential for dimerization of plectin. (A) Cell lysates of keratinocytes expressing full-length (OC1) or rodless (clone 1) plectin were subjected to gel filtration over Superose 6. The collected fractions (0–9) were analyzed by Western blot for the presence of plectin. Fractions containing the void and the 669-kDa marker protein thyroglobulin are indicated. L, lysates prior to gel filtration. (B) Cell lysates of keratinocytes expressing full-length (FL, OC1) or rodless (RL, clone 1) plectin were left untreated (–), mock treated with dimethyl sulfoxide, or incubated with increasing concentrations of the chemical cross-linker DSP. After cross-linking, the lysates were separated by SDS–PAGE under reducing and nonreducing conditions and immunoblotted for plectin. (C) Lysates of keratinocytes expressing full-length (FL, OC1) or rodless (RL, clone 1) plectin were subjected to BN-PAGE and immunoblotted for plectin. Native samples were compared with samples containing urea and/or SDS and samples generated using RIPA lysis buffer. For urea-containing samples, only one-third of the sample volume was loaded on gel. Single and double arrowheads indicate plectin monomers and dimers, respectively. Closed arrowheads, full-length plectin; open arrowheads, rodless plectin.