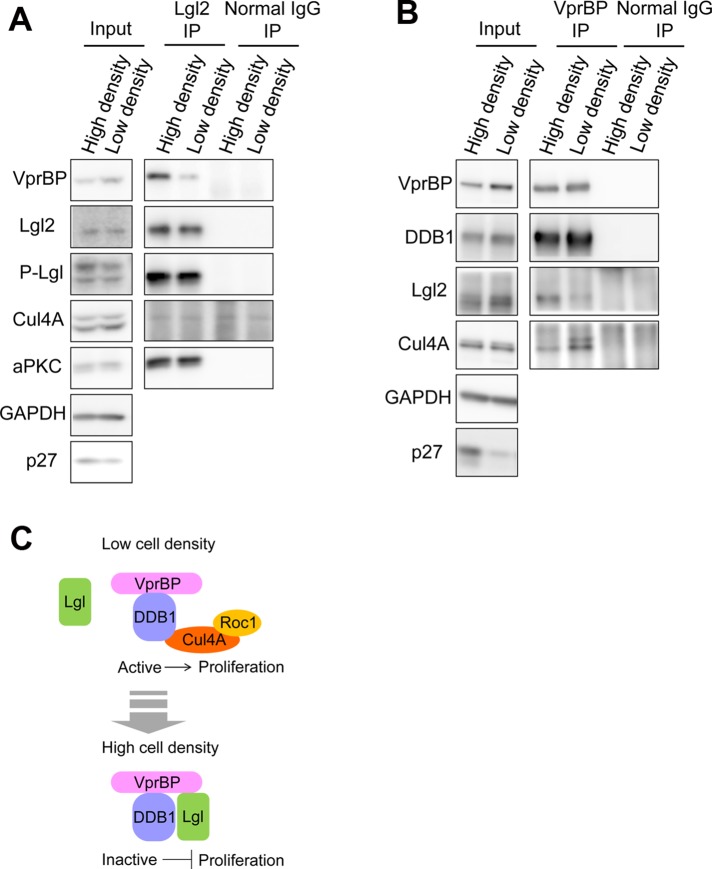
FIGURE 5:
Lgl2-VprBP-DDB1 complex was well formed with reduction of CRL4 [VprBP] complex in confluent cells. (A) Lgl2 was immunoprecipitated from the lysates of MDCK cells cultured in confluent or low-density conditions. The amount of coprecipitated VprBP was higher from confluent cells. (B) VprBP was immunoprecipitated from MDCK cell lysates cultured in confluent or low-density conditions. The amount of coprecipitated Cul4A was lower from confluent cells. (C) Hypothetical model of Lgl-mediated inhibition of the CRL4 [VprBP] complex and its downstream pathway. Lgl forms a complex with VprBP-DDB1 independently of Cul4A when cells reach confluency. Lgl-VprBP-DDB1 complex is catalytically inactive because it lacks Cul4A and Roc1, the active center that associates with E2 enzyme.