Relatively little is known about how receptor tyrosine kinase ligands can positively cooperate with BMP signaling. Primary cultures of lens cells were used to reveal an unprecedented type of cross-talk between the canonical FGF and BMP signaling pathways that regulates lens cell differentiation and intercellular coupling.
Abstract
Fibroblast growth factors (FGFs) play a central role in two processes essential for lens transparency—fiber cell differentiation and gap junction–mediated intercellular communication (GJIC). Using serum-free primary cultures of chick lens epithelial cells (DCDMLs), we investigated how the FGF and bone morphogenetic protein (BMP) signaling pathways positively cooperate to regulate lens development and function. We found that culturing DCDMLs for 6 d with the BMP blocker noggin inhibits the canonical FGF-to-ERK pathway upstream of FRS2 activation and also prevents FGF from stimulating FRS2- and ERK-independent gene expression, indicating that BMP signaling is required at the level of FGF receptors. Other experiments revealed a second type of BMP/FGF interaction by which FGF promotes expression of BMP target genes as well as of BMP4. Together these studies reveal a novel mode of cooperation between the FGF and BMP pathways in which BMP keeps lens cells in an optimally FGF-responsive state and, reciprocally, FGF enhances BMP-mediated gene expression. This interaction provides a mechanistic explanation for why disruption of either FGF or BMP signaling in the lens leads to defects in lens development and function.
INTRODUCTION
The vertebrate lens consists of a monolayer of epithelial cells and the highly elongated, crystallin-rich lens fiber cells that differentiate from them. Epithelial-to-fiber differentiation continues throughout life and takes place at the border of the anterior and posterior faces of the organ in a region referred to as the lens equator (Piatigorsky, 1981; Mochizuki and Masai, 2014). Environmental or genetic factors that perturb fiber formation cause vision-disrupting cataracts and/or microphthalmia (Reneker and Overbeek, 1996; Lovicu and Overbeek, 1998; Nishiguchi et al., 1998; Zhao et al., 2008).
As reported in >30 primary publications spanning >20 years, multiple lines of evidence indicate that one or more members of the fibroblast growth factor (FGF) family play a central role in the early stages of epithelial-to-fiber differentiation (McAvoy et al., 1999; Robinson, 2006; reviewed by Lovicu and McAvoy, 2005; Lovicu et al., 2011). It is believed that fiber formation is initiated at the lens equator because this is where epithelial cells are first exposed to the high concentrations of FGFs that diffuse out of the vitreous body. All four members of the FGF receptor (FGFR) family are expressed in the mammalian lens and share the same signaling mechanisms (Reuss and von Bohlen und Halbach, 2003). Studies in which one or more FGFRs were conditionally deleted in the mouse lens elegantly demonstrated that FGFRs are required for fiber cell differentiation and that concurrent knockout of FGFRs 1–3 is necessary to block fiber formation (Zhao et al., 2008). FGF appears to be the main activator of the extracellular signal–regulated kinase (ERK) mitogen-activated protein kinase (MAPK) in the lens in vivo (Govindarajan and Overbeek, 2001; Zhao et al., 2008).
We use as our standard experimental system dissociated cell–derived monolayer cultures of primary embryonic chick lens epithelial cells (DCDMLs; Le and Musil, 1998). Unlike central epithelial explants, the cells in DCDMLs include those from the peripheral (= preequatorial and equatorial) epithelium (Menko et al., 1984; Le and Musil, 2001a). We have shown that DCDMLs are an appropriate model system for the study of processes localized to the peripheral region of the mammalian lens in vivo, including epithelial-to-fiber cell differentiation and fiber cell–type gap junction formation, regulation, and function (Musil, 2012). As reported by Menko et al. (1984), dissociated cell–derived cultures of embryonic chick cells so closely recapitulate the in vivo process of epithelial-to-fiber differentiation that the most differentiated cells in these cultures are ultrastructurally indistinguishable from cortical fibers in the intact lens. When added to serum-free DCDMLs on day 1 of culture, purified recombinant FGF2 (or FGF1 with its cofactor heparin) at “high” concentrations (defined hereafter as ≥10 ng/ml FGF) maximally increases the expression of markers indicative of fiber cell differentiation (e.g., δ-crystallin; the beaded intermediate filament proteins CP49 and CP115) when assayed on day 6 (Le and Musil, 2001a). These levels of FGF1 and 2 also increase expression of both crystallin and noncrystallin genes in central epithelial explants prepared from either chick (Le and Musil, 2001a) or rat (McAvoy and Chamberlain, 1989) lens.
Members of the bone morphogenetic protein (BMP) family of growth factors are key regulators of early embryogenesis and the morphogenesis of a wide variety of tissues and organs (Whitman, 1998). BMP receptor 1A (ALK3) and BMP receptor II have been consistently detected in lens epithelium (Obata et al., 1999; Hung et al., 2002; de Iongh et al., 2004). We have shown that the ability of FGF to up-regulate fiber marker expression in DCDMLs is blocked when cells are cultured under conditions that prevent BMPs from binding to BMP receptors (e.g., with anti-BMP2/4/7 antibodies or with noggin; Boswell et al., 2008b; reviewed in Lovicu et al., 2011). This effect is attributable to inhibition of BMPs produced by the lens cells themselves, which act as bona fide differentiation-promoting factors. Expression of noggin in the lenses of transgenic mice (Zhao et al., 2002) resulted in a postnatal block of epithelial-to-fiber cell differentiation, consistent with (although not proof of) a role for BMP in FGF-induced fiber cell differentiation in vivo (Boswell et al., 2008b). In other studies, we showed that treating DCDMLs with purified BMP2, 4, or 7 at “high” concentrations (defined hereafter as ≥4 ng/ml BMP) induces the expression of markers of epithelial-to-fiber differentiation in a process that does not require signaling from endogenously produced FGF. This is unlike up-regulation of fiber cell differentiation in response to exogenously added FGF, which is obligatorily linked to lens-derived BMP signaling (Boswell et al., 2008b). We are not aware of a precedent for this type of nonreciprocal interaction between FGF and BMP in any system. This includes early retina/lens explants, in which exogenously added BMP cannot induce either cell cycle exit or normal equarin expression in the absence of FGF (Jarrin et al., 2012).
Growth factor signaling also regulates another process essential for lens function, gap junction–mediated intercellular communication (GJIC). Gap junctional coupling is much higher at the lens equator than at either pole (Baldo and Mathias, 1992; Mathias et al., 1997), a distribution believed to be required for lens homeostasis (Mathias et al., 1997; Donaldson et al., 2001; Sweeney et al., 2003). We reported that FGF enhances GJIC in DCDMLs in a concentration-dependent manner and that the pole-to-equator gradient in FGF signaling in the lens in vivo may be responsible for the observed asymmetry in GJIC (Le and Musil, 2001b). Up-regulation of GJIC by FGF in DCDMLs occurs before, and is not a prerequisite for, fiber differentiation, demonstrating that they are independently regulated processes. Nonetheless, the ability of FGF to up-regulate GJIC, like its effect on fiber formation, depends on signaling from lens-derived BMPs. Similar to fiber differentiation, GJIC can be enhanced in the absence of FGF signaling by culturing DCDMLs with “high” levels of BMP2, 4, or 7 (Boswell et al., 2008a).
In many nonlens systems, FGF and BMP signals act in an opposing manner to regulate a myriad of processes, including limb growth (Niswander and Martin, 1993), neural induction (Wilson and Edlund, 2001), placode formation (Sjödal et al., 2007), cranial suture closure (Warren et al. 2003), patterning of the olfactory epithelium (Maier et al., 2010), chondrogenesis (Yoon et al., 2006), and lung bud morphogenesis (Weaver et al., 2000). Although it has long been recognized that this antagonistic relationship cannot hold true in all situations (Whitman, 1998; Sapkota et al., 2007), knowledge of how FGFs and BMPs can positively cooperate is limited. In this study, we investigate the synergistic interaction between the BMP and FGF pathways that up-regulates secondary fiber differentiation and GJIC in lens cells. We present evidence that two types of mechanisms are operative. In the first, signaling from lens-derived BMPs is required to maintain lens cells in an optimally FGF-responsive state. In the second, FGF potentiates expression of BMP target genes and of BMP4.
RESULTS
BMP signaling is required to maintain lens cells in an optimally FGF-responsive state
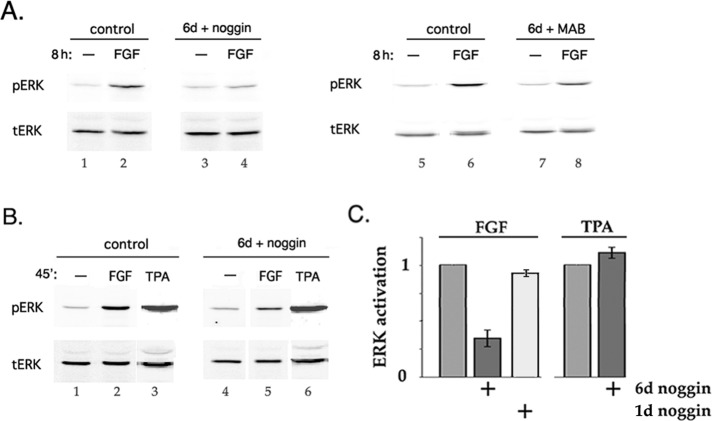
Up-regulation of markers of fiber differentiation in chick lens epithelial cell DCDML cultures in response to FGF is blocked if the cells are coincubated during the 6-d culture period with noggin (Boswell et al., 2008b), a highly specific inhibitor of binding of BMP2/4/7 to BMP receptors (Zimmerman et al., 1996; Groppe et al., 2002). One potential mechanism for this effect is that signaling by endogenously expressed, noggin-sensitive BMPs (BMP4 and/or 7; Boswell et al., 2008b) is required for the full activity of one or more key components of the FGF signaling pathway in lens cells. To test this possibility, we examined the consequences of suppressing BMP signaling with noggin on the ability of FGF2 to activate ERK, the best-characterized effector downstream of FGF in lens cells (Figure 1). Levels of FGF sufficient to stimulate fiber differentiation induce sustained (>4 h) activation of ERK (Le and Musil, 2001b; Lovicu and McAvoy, 2001). Normalized to total ERK, dually phosphorylated (activated) ERK (pERK) was increased 5.46 (±0.98)-fold after an 8-h incubation with 10 ng/ml FGF2 in otherwise untreated cells (Figure 1A, lane 2). This was reduced to 1.93 (±0.46)-fold in cultures preincubated for 6 d with noggin before addition of FGF (n = 10; lane 4). Noggin does not affect lens cell viability, proliferation, or epithelial phenotype (Boswell et al., 2008a, b), nor does it change the expression of total ERK protein (lanes 1–4; bottom). Activation of ERK in response to FGF was also decreased if BMP signaling was suppressed throughout the 6-d culture period by using a function-neutralizing anti-BMP monoclonal antibody (MAB3552) instead of noggin (Figure 1A, lanes 5–8). Control experiments demonstrated that neither noggin nor anti-BMP antibody interferes with the capacity of FGF to productively interact with its receptor (Boswell et al., 2008a). A 6-d preincubation with noggin also markedly reduced activation of ERK in response to a brief (15–60 min) exposure to 5–30 ng/ml FGF (Figure 1B; compare lane 2 to lane 5). Under the same conditions, noggin also down-regulated activation of p38 by FGF, which we show is downstream of ERK in DCDMLs (Supplemental Figure S1). Noggin did not, however, reduce activation of ERK by the FGF-unrelated protein kinase C (PKC) and ERK agonist 50 nM 12-O-tetradecanoylphorbol 13-acetate (TPA; see later discussion of Figure 4A; Chen and Kroog, 2004) (Figure 1B, lane 3 vs. lane 6), showing the specificity of the effect. Time-course studies revealed that >1-d treatment with noggin was required to significantly reduce stimulation of ERK by FGF (Figure 1C).
FIGURE 1:
Culturing lens cells with a BMP inhibitor decreases their ability to activate ERK in response to FGF. DCDMLs were cultured for 6 d with no additions (control) or with either noggin or the function-blocking anti-BMP antibody MAB3552 (MAB). Cells were then incubated for 8 h (A) or 45 min (B) with no additions (–), 10 ng/ml FGF2, or 50 ng/ml TPA, followed by Western blot analysis of activated (pERK) and total (tERK) ERK kinase. Note that chick cells express only a single ERK isoform (ERK2). (C) Summary of experiments conducted as in B. Results graphed as fold activation of ERK (normalized to total ERK) in response to FGF (n = 12) or TPA (n = 3) in cells preincubated with noggin for 6 d compared with pERK/tERK in cells without noggin preincubation. Included are results from cells preincubated with noggin for only 1 d (n = 3).
FIGURE 4:
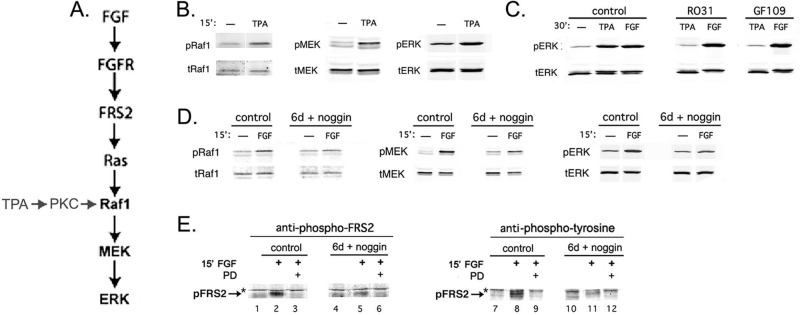
Noggin pretreatment inhibits steps upstream of ERK and downstream of FGF receptors in the canonical FGF-to-ERK signaling pathway. (A) Schematic of canonical FGF-to-ERK and TPA-to-ERK signaling pathways. (B) TPA activates Raf1 and MEK, as well as ERK. Lysates from DCDMLs incubated without (–) or with 50 ng/ml TPA for 15 min were analyzed by Western blot using antibodies that recognize either total (t) or activated (phosphorylated; p) forms of the indicated kinases. (C) Activation of ERK by TPA, but not by FGF, is downstream of PKC. Cells were incubated for 30 min without additions (control) or with 5 μM R031-8220 (R031) or GF109203X (GF109), both inhibitors of PKC. TPA (50 ng/ml) or FGF2 (5 ng/ml) was then added, and the cells were incubated for an additional 30 min before analysis for activated and total ERK. In the gel system used, activation of ERK resulted in a slight decrease in its electrophoretic mobility. (D, E) DCDMLs were cultured for 6 d in the absence (control) or presence of noggin and then treated for 15 min with or without 10 ng/ml FGF2. (D) Effect on activation of kinases. Whole-cell lysates were analyzed by Western blot using antibodies that recognize either activated or total forms of Raf1, MEK, or ERK. FGF-induced activation of Raf1 and of MEK was reduced by 86.6 ± 23% and by 72.3 ± 5.1%, respectively, in noggin-pretreated cells (n = 3). (E) Effect on activation of the FRS2 adaptor. Whole-cell lysates were analyzed by Western blot using antibodies against either phospho-FRS2 (Tyr-196) or phosphotyrosine. Phosphorylated FRS2 was not detected when cells were coincubated with FGF and the FGFR blocker PD173074 (PD). Equal protein loads in each lane. Asterisk, nonspecific band.
Next we inhibited BMP signaling at the level of BMP receptors using dorsomorphin, a selective small-molecule inhibitor of ligand-activated type I BMP receptors (BMPRs; Yu et al., 2008; Kamaid et al., 2010). We confirmed that a 30-min preincubation with 5 μM dorsomorphin completely blocked BMP4 from activating its downstream effectors Smad1/5 in DCDMLs but did not prevent activation of Smad3 or ERK in response to transforming growth factor β (TGFβ) or FGF, respectively (Figure 2A). To test the effect of dorsomorphin on expression of lens fiber cell markers, we cultured DCDMLs with dorsomorphin for 6 d in the presence of BMP4 or FGF2. Dorsomorphin blocked up-regulation of δ-crystallin and CP49 by either growth factor, without detectable deleterious effects on the cells (Figure 2B). A 6-d incubation with dorsomorphin also down-regulated the ability of subsequently added FGF to activate ERK by 65% (±7.7; n = 3; Figure 2C). Thus dorsomorphin has the same inhibitory effect as noggin on FGF-induced lens fiber differentiation and ERK activation, further supporting the role of the canonical BMP signaling pathway in these events.
FIGURE 2:
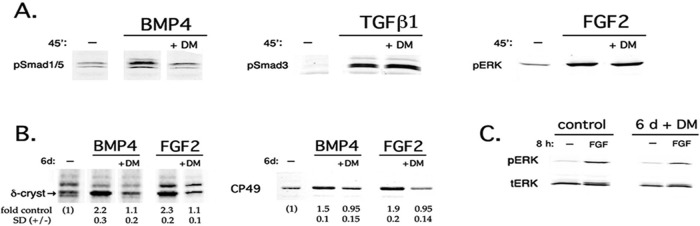
BMP-dependent FGF signaling in lens cells requires active BMP receptors. (A) Dorsomorphin is a specific inhibitor of BMP signaling in DCDMLs. Cultures were treated for 45 min without factors (–) or with 5 ng/ml BMP4, 4 ng/ml TGFβ1, or 10 ng/ml FGF2. Where indicated, cells were pretreated with 5 μM dorsomorphin (DM) before addition of the factor. Whole-cell lysates were probed with antibodies specific for the phosphorylated (activated) forms of Smad1/5, Smad3, or ERK. Representative of three independent experiments; inhibition of either Smad3 or ERK activation never exceeded 20%. (B, C) Dorsomorphin blocks both BMP- and FGF-induced processes in DCDMLs. DCDMLs were incubated without growth factor or with 10 ng/ml BMP4 or FGF2 in either the absence or presence of dorsomorphin as indicated. (B) After 6 d of culture, cells were assayed for synthesis of the fiber differentiation markers δ-crystallin (by [35S]methionine labeling) and CP49 (by quantitative anti-CP49 Western blotting). Fold increase over control ± SD given for each condition (n = 5). (C) Cultures were assessed for their ability to activate ERK in response to an 8-h incubation with FGF2 as in Figure 1A. Percentage decrease in activation of ERK in response to FGF in dorsomorphin-treated cells relative to cells not cultured with dorsomorphin was 65 ± 7.7% (n = 3).
Experiments using purified FGF in chick lens cells or in the rat lens epithelial explant system have been conducted with either FGF2 or FGF1. Although the identity of the FGF family member(s) essential for lens fiber differentiation in vivo is unknown, Robinson (2006) concluded that the most likely candidate in the mammalian and avian lens is FGF9. When assayed as described for FGF2, ≥10 ng/ml recombinant, purified FGF9 also up-regulated expression of markers of fiber differentiation (δ-crystallin; CP49; Figure 3A) and induced sustained (>8 h) activation of ERK (Figure 3B). As was the case for FGF2, these FGF9-mediated processes were inhibited by noggin, demonstrating a similar requirement for endogenous BMP signaling. Normalized to total ERK, fold activation of ERK in response to FGF9 dropped from 3.3× (±0.6) in the absence of noggin to 1.2× (±0.17) after a 6-d preincubation with noggin (n = 3), an extent of down-regulation very similar to that obtained with FGF2 (Figure 1).
FIGURE 3:
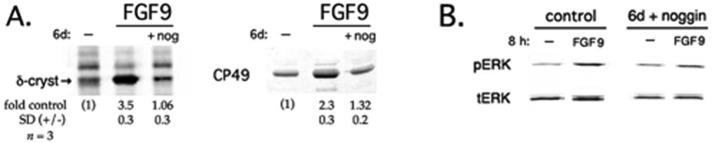
Signaling by FGF9 also requires lens-endogenous BMPs. (A) DCDMLs were cultured for 6 d with or without (–) 20 ng/ml FGF9 in either the absence or presence of noggin (nog) as indicated. Up-regulation of markers of fiber differentiation was assessed as in Figure 2B. (B) DCDMLs were cultured for 6 d without (control) or with noggin before being incubated with no additions (–) or 20 ng/ml FGF9 for 8 h. Activation of ERK assessed as in Figure 1.
Having demonstrated that our results with noggin and FGF2 can be reproduced using other BMP and FGF signaling effectors, we next addressed the mechanistic basis for the synergistic interaction between the FGF and BMP pathways. We have shown that FGF stimulates ERK in lens cells via the canonical FGFR → Ras → Raf1 → MAPK kinase (MEK) → ERK signaling module (Boswell et al., 2009; Figure 4A). In most cell types, TPA activates PKC, which in turn stimulates Raf1, MEK, and then ERK (Kolch et al., 1993; Ueda et al., 1996). This is also likely to be the case in DCDMLs, as evidenced by the ability of TPA to enhance the phosphorylation (activation) of all three of the last-named kinases (Figure 4B) and the finding that PKC inhibitors block activation of ERK induced by TPA but not by FGF (Figure 4C). The finding that a 6-d incubation with noggin decreases phosphorylation of ERK in response to FGF but not to TPA (Figure 1) suggests that noggin reduces the activity of a component upstream of Raf1 in the canonical FGF-to-ERK signaling pathway. Consistent with this possibility, a 6-d preincubation with noggin greatly reduced phosphorylation of Raf1 and MEK in response to subsequently added FGF but not the total levels of either kinase (Figure 4D). FRS2 is a docking protein constitutively bound to FGF receptors that mediates most of the downstream effects of FGF-FGFR interaction, including activation of Ras and the ERK pathway (Eswarakumar et al., 2005; Madakashira et al., 2012; Li et al., 2014). We found that consistently less FRS2 became activated (tyrosine phosphorylated) in response to a 15-min treatment with FGF2 in noggin-preincubated cells than in controls, as assessed with a phosphospecific (Tyr-196) antibody against FRS2 (43.5% inhibition ± 12.1; n = 4; Figure 4E, compare lane 2 with lane 5). The phospho-FRS2 band was not detected when cells were treated with FGF in the presence of the FGFR blocker PD173074, confirming its identity. The block in FRS2 activation was also detectable using a total anti-phosphotyrosine antibody (Figure 4E, lane 8 vs. lane 11). Taken together, these results show that noggin inhibits FGF-to-ERK signaling upstream of FRS2 activation, supporting a role for endogenous BMP signaling at the level of FGF receptors.
If endogenous BMP signaling were required for FGF receptor function, then noggin pretreatment would be expected to inhibit processes downstream of FGF binding even if they are not mediated by FRS2 and ERK. This was confirmed in a series of experiments using a reporter construct driven by upstream elements from the gene encoding mouse αA crystallin, a marker of fiber differentiation whose expression in mouse lens is not dependent on FRS2 or ERK signaling (Li et al., 2014; Figure 5). The DCR1 lens-specific enhancer confers on the DCR1-αA-promoter–enhanced green fluorescent protein (EGFP) construct the ability to be up-regulated by FGF2 in rat lens central epithelial explants (Yang et al., 2006). Addition of FGF at concentrations that induce fiber formation (lane 2), but not below (lane 6), also increased DCR1-αA-promoter-EGFP levels in transiently transfected DCDMLs by 3.47 (±0.35)-fold (n = 4) over a 6- to 9-d period. As expected, this up-regulation was blocked by the FGFR kinase inhibitor PD173074 (lane 3). In contrast, the MEK inhibitor UO126 had no significant effect (lane 4), indicating that expression of the reporter, like that of endogenous crystallin genes in mammalian (Lovicu and McAvoy, 2001) and avian (Le and Musil, 2001a) lens cells, does not require ERK activity. Up-regulation of DCR1-αA-promoter-EGFP by FGF was, however, completely blocked by coculture with noggin (lane 5). Of note, synthesis of DCR1-αA-promoter-EGFP was also stimulated 3.4-fold by 5 ng/ml BMP4 in a UO126-insensitive, noggin-inhibitable manner (lanes 7–9); we did not test the effect of BMP4 + PD173074. Thus the mouse DCR1-αA-promoter-EGFP construct behaved like endogenously expressed δ-crystallin in DCDMLs, in that its expression was increased over a >3-d period by either BMP4 or differentiation-inducing levels of FGF2 in a process that is independent of ERK but sensitive to noggin.
FIGURE 5:
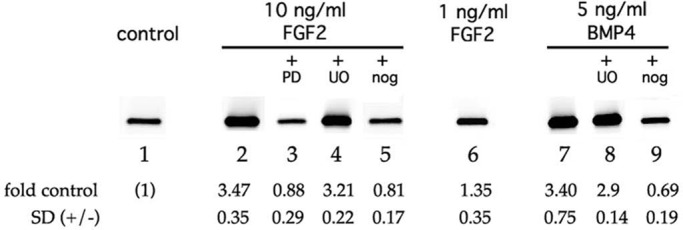
Noggin pretreatment inhibits FRS2- and ERK-independent FGF signaling. DCDMLs were transfected with the DCR1-αA-promoter-EGFP reporter construct and cultured for 9 d with no additions (control), 10 ng/ml FGF2, 1 ng/ml FGF2, or 5 ng/ml BMP4. Where indicated, the cells were incubated with growth factor in the presence of PD173074 (PD), UO126 (UO), or noggin (nog). Expression of EGFP was assessed by Western blot analysis (2 μg total cell lysate/lane). Fold increase over control ± SD given for each condition (n ≥ 3).
In knockout mice, lens fiber formation is blocked when the expression of FGF receptors 1, 2, and 3 is concurrently reduced by >50% (Zhao et al., 2008). The simplest explanation for how noggin inhibits FGF from up-regulating fiber differentiation would be that noggin phenocopies these conditions. Repeated attempts to quantitatively detect FGF receptor protein in DCDMLs were unsuccessful, likely due to low levels of endogenous expression. However, no decrease in either FGFR1 or FGFR3 was detected by real-time quantitative reverse transcription PCR (qRT-PCR) in DCDMLs treated with noggin for 7 d (Table 1A; FGFR2 is not expressed in chick lens; Walshe and Mason, 2000). Possible mechanisms by which noggin could reduce FGF receptor function but not expression are examined later (see Discussion).
TABLE 1:
Expression of mRNA for the indicated test gene as measured by qRT-PCR (TaqMan), using GAPDH for normalization.
| Id1 | FGFR1 | FGFR3 | BMP4 | BMP7 | ||
|---|---|---|---|---|---|---|
| A | 7 d noggin | 1.5 ± 0.06, n = 6 | 1.3 ± 0.4, n = 6 | |||
| B | 5 h BMP4 | 42.5 ± 7.5, n = 5 | ||||
| 5 h FGF2 | 4.7 ± 1.4, n = 11 | 4.07 ± 0.9, n = 7 | 0.7 ± 0.14, n = 7 | |||
| 5 h CHX | 150.5 ± 3.3, n = 4 | |||||
| 5 h FGF2 + CHX | 277.5 ± 16.1, n = 4 | |||||
| C | 5 h DRB + FGF2 | 4.1 ± 0.5, n = 2 | 1.2 ± 0.4, n = 2 |
Data are expressed as fold change over untreated cells (A, B) and DRB-only cells (C) in the same experiment. DCDMLs were preincubated with 75 μM DRB for 30 min before addition of FGF2.
FGF up-regulates BMP-mediated gene expression in DCDMLs
Having shown that BMP signaling promotes processes downstream of FGF in lens cells, we next addressed whether the converse was also true. As reported in several non–lens cell types (Hollnagel et al., 1999), a 5-h treatment of DCDMLs with 5 ng/nl BMP4 strongly increased the level of mRNA for Id1, a well-known direct transcriptional target of canonical, Smad-mediated BMP signaling (Katagiri et al., 2002; Lopez-Rovira et al., 2002; ten Dijke et al., 2003; Table 1A). Remarkably, Id1 expression was also increased by 10 ng/ml FGF, albeit to a lesser extent ([4.7 ± 1.4]-fold relative to GAPDH measured in the same sample by qRT-PCR in 11 samples from four independent experiments; Table 1B). This is in contrast to several other nonneuronal cell types, in which FGF does not affect (or reduces) Id1 message levels (Rozenblatt-Rosen et al., 2002; Reinhold et al., 2004; Rice et al., 2005; Xu et al., 2005).
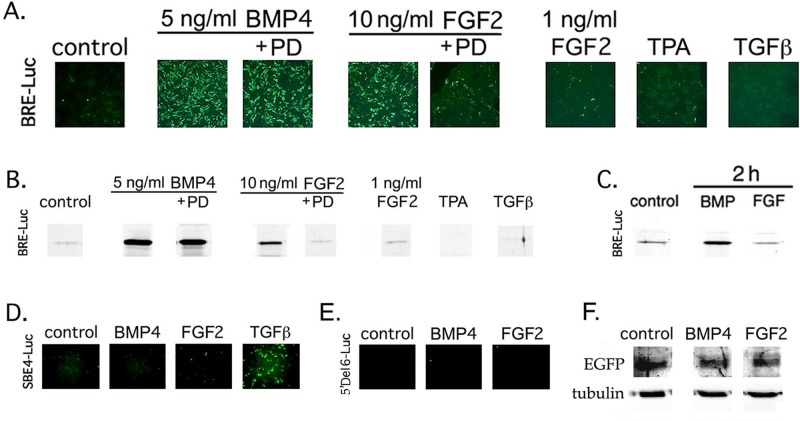
In addition to BMP response elements (BREs), the Id1 promoter contains binding sites for non-Smad transcriptional regulators that could influence the strength and/or kinetics of Id1 expression (Nehlin et al., 1997; Korchynskyi and ten Dijke, 2002). To demonstrate more definitively that FGF can promote gene expression directly downstream of the canonical BMP signaling pathway in lens cells, we transiently transfected DCDMLs with a luciferase reporter driven exclusively by BREs from mouse Id1. Expression of BRE-Luc has been extensively documented to require activation of Smad 1/5 and to be directly proportional to the amount of Smad signaling in multiple cell types (Korchynskyi and ten Dijke, 2002; Monteiro et al., 2004). As detected by anti-luciferase immunostaining, ∼70% of the cells in confluent regions of DCDML cultures expressed BRE-Luc after incubation with 5 ng/ml BMP4 for ≥5 h (Figure 6C). Given that the efficiency of transient transfection of DCDMLs is also ∼70% (Boswell et al., 2009), it appears that all of the transfected cells are transcriptionally responsive to BMP. As expected, expression of BRE-Luc was abolished if cells were cultured with BMP4 in the presence of noggin (Figure 6D). Unlike BMP4, the function of BMP6 is not effectively inhibited by noggin (Song et al., 2010). Noggin had no effect on BRE-Luc induction in response to BMP6 (Figure 6G), demonstrating that noggin is acting by binding to its cognate BMPs instead of by nonspecifically preventing BRE-Luc synthesis, regardless of the stimulus. As expected, up-regulation of BRE-Luc by either BMP species was blocked by the BMP receptor inhibitor dorsomorphin (Figure 6, E and H).
FIGURE 6:
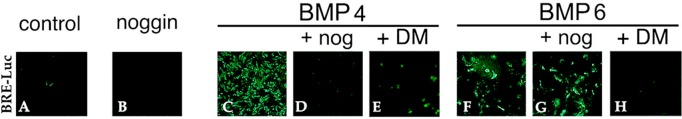
Up-regulation of the BMP signaling reporter BRE-Luc by BMPs is specifically blocked by noggin and dorsomorphin. DCDMLs were transfected with the BRE-luciferase reporter construct BRE-Luc and cultured for 2 d with no additions (control), noggin, 5 ng/ml BMP4, 10 ng/ml BMP6, or the indicated BMP plus either noggin or dorsomorphin. Fixed cultures were immunostained with anti-luciferase antibodies, and confluent regions of the monolayer were imaged. See Figure 8C for Western blot quantification of the effect of noggin and dorsomorphin on BRE-luciferase protein levels.
Expression of BRE-Luc has been reported to be insensitive to FGF in several non–lens cell types (Monteiro et al., 2004; Logeart-Avramoglou et al., 2006; Zilberberg et al., 2007), as expected, given that FGF signals via a different set of receptors and effectors than BMP. We reproduced this result in fibroblastic 10 t1/2 cells, in which BMP4, but not FGF2, induced expression of BRE-Luc despite robust activation of ERK in response to the same levels of FGF (Supplemental Figure S3). In contrast, FGF markedly increased anti-luciferase immunostaining in transiently transfected DCDMLs, although less intensely and more slowly than BMP4 (Figure 7, A–C). As quantitated by Western blot, 10 ng/ml FGF2 induced a 10.67 (±4.09)-fold (n = 10) increase in BRE-Luc levels at 22 h, compared with a 123.2 (±17.9)-fold increase with 5 ng/ml BMP4 (Figure 7B). Up-regulation of BRE-Luc by FGF, but not by BMP, was blocked by the highly selective inhibitor of FGFR kinase activity PD173074. In chick (Le and Musil, 2001a) and mammalian (McAvoy and Chamberlain, 1989) lens cells, FGF up-regulates fiber differentiation only at levels >1 ng/ml. Enhancement of BRE-Luc expression by FGF showed similar concentration dependence. Expression of BRE-Luc was not stimulated by TPA or TGFβ1, the latter a member of the same superfamily of growth factors as BMPs. Conversely, TGFβ1, but neither BMP nor FGF, induced expression of a TGFβ- and Smad3- specific reporter, SBE4-Luc (Zawel et al., 1998; Figure 7D).
FIGURE 7:
FGF specifically increases expression of the BMP signaling reporter BRE-Luc in lens cells. (A–E) DCDMLs were transfected with plasmids encoding BRE-Luc (A–C), SBE4-Luc (D), or 5′ Del 6 Id1-Luc (E) and cultured with no additions (control), 5 ng/ml BMP4, 10 ng/ml FGF2, 1 ng/ml FGF2, 4 ng/ml TGFβ1, or 50 nM TPA for 22 h (A, B, D, E), or 2 h (C). Where indicated, the FGFR inhibitor PD173074 (PD) was also present. Cultures were either fixed and immunostained with anti-luciferase antibodies (A, D, E) or lysed and equal amount of total cell protein analyzed by Western blot using the same antibody (B, C). Representative of three or more independent experiments. (F) Experiments in which a plasmid encoding constitutively expressed EGFP was cotransfected with 5′ Del 6 Id1-Luc at a ratio of 1:4 demonstrated equal levels of expression of EGFP when normalized to either total protein or tubulin, indicating that the lack of expression of 5′ Del 6 Id1-Luc in E was not due to a lack of cell transfection.
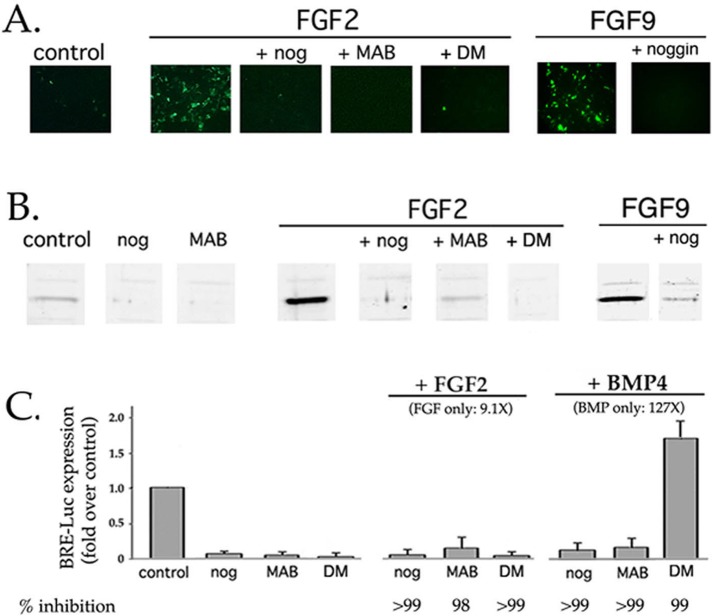
Neither BMP nor FGF induced the expression of a mouse Id1 minimal promoter-Luc construct that lacks BREs (5′ Del 6; Tournay and Benezra, 1996; Figure 7E). If BREs are both necessary and sufficient for FGF to drive BRE-Luc expression, and if BMP-activated Smad1/5 are the transcriptional regulators that bind to BREs, then how does FGF enhance BRE-Luc expression? The simplest explanation is that FGF acts by promoting canonical Smad1/5 signaling initiated by endogenous BMP4 and/or 7. If so, then the ability of FGF to induce BRE-Luc expression should be inhibited by noggin. This was the result obtained for both FGF2 and FGF9 (Figure 8). Up-regulation of BRE-Luc by FGF was also abolished by the BMPR inhibitor dorsomorphin or the function-neutralizing anti-BMP antibody MAB3552. Similar results were obtained with another BRE-containing transcriptional reporter, pID1SB-Luc (Nehlin et al., 1997; Supplemental Figure S2).
FIGURE 8:
Up-regulation of BRE-Luc expression by FGF requires signaling from endogenously expressed, noggin-sensitive BMPs. DCDMLs were transfected with BRE-Luc and cultured with no added growth factor (control), 10 ng/ml FGF2, 20 ng/ml FGF9, or 5 ng/ml BMP4. Incubations were conducted in either the absence or presence of noggin (nog), the anti-BMP antibody MAB3552 (MAB), or dorsomorphin (DM). After 22 h, cultures were either fixed and immunostained with anti-luciferase antibodies (A) or lysed and equal amount of total cell protein analyzed by Western blot using the same antibody (B). (C) Summary of experiments conducted as in B, compared with results obtained in the same experiments from cells cultured for 22 h with 5 ng/ml BMP4. Results graphed as fold increase in luciferase levels relative to untreated control transfectants (n = 3). The percentage inhibition relative to growth factor alone is included. The low level of anti-luciferase immunoreactivity in untreated control transfectants is due to endogenous BMP4/7 signaling, as demonstrated by its near absence in cells treated with noggin, MAB3552, or dorsomorphin alone. Immunofluorescence images showing the effect of noggin and dorsomorphin on BMP4-induced BRE-Luc expression are presented in Figure 6.
Role of BMP signaling in lens-cell gap junctional communication and proliferation
We reported that exogenously added FGF2 or BMP4 increases the gap junction–mediated intercellular transfer of Lucifer yellow in DCDMLs in 2 d (Boswell et al., 2008a). Up-regulation of gap junctional coupling by either factor is blocked by noggin or anti-BMP function-neutralizing antibodies (Boswell et al., 2008a); similar results were observed with FGF9 (Figure 9A). Dorsomorphin also blocked BMP- or FGF-induced GJIC within 48 h (Figure 9A).
FIGURE 9:
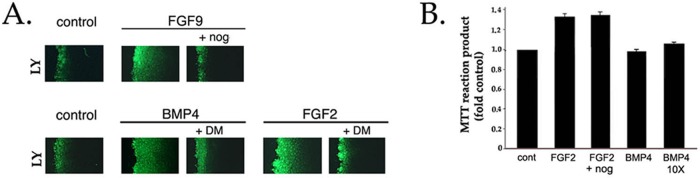
Up-regulation of gap junctional communication, but not of cell proliferation, by FGF requires signaling from endogenously expressed BMPs. (A) DCDMLs were incubated without growth factor (control) or with 10 ng/ml BMP4, 10 ng/ml FGF2, or 20 ng/ml FGF9 in either the absence or presence of noggin or dorsomorphin as indicated. After 2 d of culture, cells were assayed for gap junction–mediated intercellular spread of Lucifer yellow (LY) using the scrape-load dye transfer assay. Typical of three independent experiments. (B) DCDMLs were plated at low density (0.7 × 105 cells/well) and incubated with no additions (cont), 2 ng/ml FGF2, 2 ng/ml FGF2 + noggin, 0.2 ng/ml BMP4, or 20 ng/ml BMP4 (10X). After 2 d, the MTT assay was used to colorimetrically assess cell proliferation. Data expressed as fold OD 570 nm experimental/OD 570 nm untreated control. n = 3.
As in mammalian systems (Lovicu and McAvoy, 2001), low levels of FGF stimulate cell proliferation in DCDMLs (Le and Musil, 2001a). In contrast to gap junctional coupling, however, this process cannot be stimulated by exogenous addition of 0.2–20 ng/ml BMP4 (Figure 9B). Moreover, noggin did not prevent 2 ng/ml FGF2 from enhancing cell proliferation, as assessed using a standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Figure 9B). Thus BMP signaling is not necessary for stimulation of lens cell proliferation by FGF nor is it sufficient to do so on its own, demonstrating that BMP does not affect all processes downstream of FGF.
Effect of FGF on BMP expression
One mechanism that could explain why noggin blocks the ability of FGF to up-regulate fiber differentiation and GJIC (both of which can also be stimulated by exogenously added BMP) but not cell proliferation (which is BMP insensitive; Figure 9B) would be if FGF enhances the synthesis of BMPs. We used real-time qRT-PCR to examine the effect of FGF on the message levels of BMP7 and BMP4, noggin-sensitive BMPs expressed by both chick (Boswell et al., 2008b) and mammalian (Thut et al., 2001; Hung et al., 2002; Bakrania et al., 2008) lens cells. We found in three of three independent experiments (seven total samples) that 10 ng/ml FGF2 modestly decreased the levels of BMP7 by ∼30 ± 14% within 5 h. In contrast, transcripts for BMP4 were increased 4 (±0.96)-fold within the same samples (Table 1B).
Increased synthesis of BMP4 may contribute to, but cannot be solely responsible for, up-regulation of BMP target gene expression by FGF in lens cells
As reported in other cell types (Tournay and Benezra, 1996; Katagiri et al., 2002; Lopez-Rovira et al., 2002), treatment of DCDMLs with cycloheximide (CHX; 20 μg/ml) alone greatly increased Id1 transcript levels (Table 1B), possibly by reducing the amount of a short-lived protein required for Id1 mRNA degradation. Id1 message levels were higher, however, if cells were treated with FGF and CHX than with CHX alone. This is in contrast to several reports in other systems in which CHX completely abolished FGF-induced transcriptional events (Isaac et al., 2000; Li et al., 2003; Vargas et al., 2005). Further evidence that stimulation of Id1 by FGF does not require new gene expression was obtained using the transcriptional inhibitor 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB). In the same samples in which DRB prevented FGF from increasing the level of BMP4 transcripts, Id1 message levels were still elevated approximately fourfold relative to samples from cells treated with DRB alone. Taken together, the findings in Table 1, B and C, demonstrate that augmentation of Id1 by FGF can occur in the absence of new BMP4 synthesis. We conclude that FGF can up-regulate BMP target genes by at least two mechanisms: 1) by increasing the de novo expression of BMP4, which then stimulates the canonical BMP/Smad signaling pathway, and 2) by more directly affecting BMP target gene transcripts (e.g., Id1), possibly by stabilizing them against degradation.
DISCUSSION
Molecular mechanisms by which receptor tyrosine kinase ligands such as FGF inhibit BMP signaling were first elucidated nearly two decades ago (Kretzschmar et al., 1997). How RTK ligands can positively cooperate with BMP—a less common, but as physiologically important, occurrence—is poorly understood. We show here that in lens cells, basal signaling from lens-derived, noggin-sensitive BMPs potentiates signaling by FGF. Conversely, FGF enhances the ability of lens-endogenous BMP to stimulate expression of BMP target genes. Our findings explain why levels of endogenous BMP too low to induce fiber differentiation or GJIC in unstimulated lens cells are nonetheless required for up-regulation of these essential processes by FGF. FGF cannot activate BMP receptors, nor can BMP engage FGFRs. In what follows, we discuss possible mechanisms for this synergistic interaction.
Potentiation of FGF signaling by BMP
We showed that a 6-d, but not a 1-d, exposure of DCDML cultures to the BMP blocker noggin inhibits FGF-induced signaling pathways (Figure 1). This includes the first step downstream of FGF receptor activation in most FGF-stimulated signaling pathways, namely phosphorylation of FRS2 (Figure 3B). The simplest explanation for why such a prolonged pretreatment with noggin is required would be if blocking endogenous BMP signaling reduced the expression of FGFRs at the transcript level. We did not, however, find any evidence for this by real-time RT-PCR (Table 1A). Noggin may instead induce changes in the expression of other genes known to affect FGF signaling. One possibility consistent with our findings is that lens-endogenous BMP signaling is required for the production or function of heparin sulfate proteoglycans that serve to enhance FGF/FGFR affinity and/or prolong receptor half-life (Knights and Cook, 2010). Genetic ablation of the heparin sulfate biosynthetic gene Ndst1 disrupts binding of multiple FGFs to FGFRs in the early lens, leading to decreased activation of ERK and defects in lens fiber differentiation (Pan et al., 2006). Another mechanism by which endogenous BMP could promote FGFR signaling at the transcriptional level would be by down-regulating an inhibitor of FGFR function such as c-Cbl or Sef (Wong et al., 2002; Kovalenko et al., 2003; Cho et al., 2004; Xian et al., 2007). It is also conceivable that inhibiting BMP signaling induces the expression of a microRNA (miRNA) that decreases the translation of FGFRs. Noggin–up-regulated miRNAs have been reported in keratinocytes, as has miRNA-mediated reduction of FGFR at the protein, but not transcript, level (Xu et al., 2011). By reducing FGFR activation to below the threshold needed to promote fiber differentiation (McAvoy and Chamberlain, 1989; Le and Musil, 2001a) and gap junctional communication (Le and Musil, 2001b), any of the aforementioned mechanisms would explain how noggin blocks the ability of FGF to enhance GJIC, as well as the expression of a wide range of fiber cell differentiation–associated genes, irrespective of their transcription factor requirements or dependence on ERK activation.
Why does noggin block the ability of FGF to up-regulate lens fiber marker expression and GJIC but not cell proliferation? Even after a 6-d preincubation, noggin only partially reduces FGF signaling, as indicated by the ∼65% reduction in FGF-induced activation of ERK (Figure 1). It is possible that this lower level of FGF signaling is sufficient to support normal cell proliferation in response to 2 ng/ml FGF but not fiber differentiation and GJIC, which require higher levels of FGF. Alternatively or in addition, stimulation of cell proliferation by FGF may not require noggin-sensitive changes in gene expression. Because DCDMLs do not tolerate prolonged (>24 h) exposure to transcriptional or translational inhibitors, the role of de novo gene expression in FGF-mediated cell proliferation, differentiation, or GJIC cannot be directly assessed.
Potentiation of BMP signaling by FGF
It is unlikely that FGF potentiates lens-endogenous BMP signaling by increasing the synthesis of BMP receptors, because addition of high (≥5 ng/ml) levels of exogenous BMP4 or 7 increases Smad1 activation in otherwise unstimulated DCDMLs in <15 min, indicating that the number of BMPRs present under basal conditions is more than sufficient to mediate signaling by the lower levels of endogenous BMP. Instead, our data are consistent with two alternative, non–mutually exclusive mechanisms. First, FGF could enhance the signaling capacity of BMP and/or BMPRs by, for example, increasing the release of BMP from the extracellular matrix, increasing the engagement of a BMP coreceptor, or down-regulating a BMPR phosphatase (e.g., Dullard; Satow et al., 2006) or another inhibitor of BMP signaling (Reinhold et al., 2004). Second, FGF could increase the synthesis of a noggin-sensitive BMP to levels that functionally mimic exogenous addition of BMP2, 4, or 7. Autoactivation of BMP4 expression has been reported in many systems (Schuler-Metz et al., 2000; Wijgerde et al., 2005; Kozmikova et al., 2013; Wang et al., 2013). Although our finding that FGF increases the level of BMP4 mRNA within 5 h is consistent with the second mechanism, it does not rule out the first. Indeed, increasing the expression of BMP4 cannot be the sole mechanism by which FGF promotes BMP target gene expression, given that FGF still increases Id1 message levels if synthesis of BMP4 is blocked by CHX (Table 1B) or DRB (Table 1C). Because we do not yet have a means to block up-regulation of BMP4 by FGF without also abolishing basal BMP4 expression, we cannot experimentally determine the role of FGF-induced stimulation of BMP4 synthesis.
Comparison to mammalian systems
Wolf et al. (2013) conducted an mRNA expression profiling analysis of the effects of FGF on central epithelial explants generated from the lenses of postnatal rats. They reported that FGF2 up-regulated the expression of Id1 by approximately twofold within 4 h and that of BMP4 by approximately fivefold within 12 h. BMP7 transcripts were reduced by ∼50% over the same period. These findings mirror our results in chick lens cells and further substantiate the fundamental similarity between bird and mammalian lenses in growth factor signaling. Although the reason for the slower induction of BMP4 in rat explants compared with DCDMLs is unknown, it could be due to differences in growth factor sensitivity and/or signaling between central and peripheral lens epithelial cells (Richardson et al., 1992, 1993; Boswell et al., 2008b). Wolf et al. (2013) also reported that FGF increases the message levels of two other BRE-containing direct BMP target genes, Id2 and Id3 (Shin et al., 2013), as well as of BMP2. Because the last is not expressed in chick (Boswell et al., 2008b) or human (Dawes et al., 2007) lens cells, the physiological significance of BMP2 in the lens is unclear.
In mice, germline knockout of BMP7 or BMP4 (Furuta and Hogan, 1998; Wawersik et al., 1999) prevents the formation of a lens, as does conditional deletion of both ALK3 (Bmpr1a) and ALK2 (Acvr1) BMP receptors in surface ectoderm at embryonic day 9.5 driven by LeCre (Rajagopal et al., 2009). Conditional deletion of only ALK3 with LeCre resulted in a smaller lens with defects in fiber cell differentiation (Beebe et al., 2004). Transgenic overexpression of noggin in the lens at a later development stage leads to a block in secondary fiber cell formation at the lens equator (Boswell et al., 2008b). Both lens placodes (Rajagopal et al., 2009) and the equatorial region in older lenses (Beebe et al., 2004; Rajagopal et al., 2007) show strong nuclear staining for phospho-Smad1/5, indicative of ongoing BMP signaling. Taken together, these findings are consistent with BMP affecting both early and late lens development via the canonical Smad 1/5 pathway, as is the case in many other organs. It was therefore surprising that Rajagopal et al. (2009) reported that conditional deletion of both Smad1 and Smad 5 using LeCre does not block lens formation. The simplest interpretation of these results is that BMP-dependent Smads are not required for the earliest stages of lens development. This does not, however, appear to be the case for secondary fiber cell differentiation, given that images from P3 Le-Cre Smad1/5 double-knockout mice published in Rajagopal et al. (2009) show a clearly abnormal lens, with retention of nuclei in fiber cells and what appears to be a monolayer of undifferentiated epithelial cells encircling the fiber cell mass. Both of these defects in secondary fiber differentiation are also present in P14 mice that overexpress noggin in the lens (Boswell et al., 2008b). The fact that epithelial cell proliferation is not obviously compromised in Smad1/5 double-knockout (Rajagopal et al., 2009) or noggin-overexpressing (Boswell et al., 2008b) lenses is consistent with our finding in DCDMLs that FGF up-regulates cell proliferation in a BMP-independent manner (Figure 9B).
Model
We propose that in vivo, lens epithelial cells are continuously exposed to the low levels of BMP that they endogenously produce. One downstream effect of this autocrine/paracrine BMP signaling is to maintain lens cells in a state in which they can be optimally activated by high levels of FGF, most likely by promoting the function of FGFRs. Epithelial cells first encounter these levels of FGF at the lens equator, where they gain access to diffusible factors from the vitreous body (Schulz et al., 1993; Le and Musil, 2001a). Vitreous-derived FGF then potentiates endogenous BMP signaling to the extent that expression of one or more critical BMP target genes is enhanced, leading to up-regulation of fiber differentiation and gap junctional coupling. This process is likely facilitated by enhanced expression of BMP4 by FGF. Increased BMP-mediated transcription at the lens equator could explain why activated Smad1 and its binding partner Smad4, localized in the cytosol in central epithelial cells, redistribute into the nucleus in elongating lens fiber cells in both mouse and chick lens (Rajagopal et al., 2007). In this view, BMP signaling is as important as FGF signaling in normal secondary fiber cell differentiation and function. Our findings may also shed light on other important physiological processes in which BMP and FGFs act agonistically, such as nephrogenesis (Dudley et al., 1999), specification of the ciliary body (Dias da Silva et al., 2007), lens induction (Faber et al., 2001), and primary lens fiber cell differentiation (Jarrin et al., 2012).
MATERIALS AND METHODS
Materials
Recombinant bovine FGF2, human FGF9, human TGFβ1, mouse noggin/Fc chimera, human BMP4, human BMP6, and anti-BMP antibody (MAB3552) were from R&D Systems (Minneapolis, MN). The following antibodies were all purchased from Cell Signaling Technology (Danvers, MA): anti–phospho-p44/42 MAPK E10 mouse monoclonal (#9106), anti–total p44/42 MAPK (#9102), anti–phospho-p38 (#9211), anti–phospho-MEK1/2 (#9154), anti–phospho Raf-1 (#9427), anti–phospho-FRS2-α(Tyr-196) (#3864), and anti–phospho-Smad1 (Ser463/465)/Smad5 (Ser463/465) (#9511). Other antibodies used were as follows: for CP49, rabbit anti-mouse CP49 polyclonal serum (#899 or #900; both generous gifts of Paul FitzGerald, University of California, Davis, CA); for phospho-tyrosine, 4G10 (a kind gift from Brian Druker, Oregon Health and Science University, Portland, OR); for luciferase, #G745A from Promega (Madison, WI); for GFP, JL-8 from Clontech (Mountain View, CA); for phospho-Smad3, ab51451 from Abcam (Cambridge, MA); for total Smad 1/5, ab75273 from Abcam; for total Raf-1, sc-7267 from Santa Cruz Biotechnology (Santa Cruz, CA); for total p38, sc-535 from Santa Cruz; and for total MEK, M17030 from Transduction Labs (Lexington, KT). UO126 (used at 15 μM), PD173074 (100 nM), and dorsomorphin (5 μM) were from Calbiochem (La Jolla, CA). All other reagents, including TPA, were from Sigma-Aldrich (St. Louis, MO).
Cell culture and treatments
Cultures were prepared from E10 chick lenses and plated at 1.2 × 105 cells/well onto laminin-coated 96-well tissue culture plates as previously described in Le and Musil (1998). Cells were cultured in the absence of serum in M199 medium plus BOTS (2.5 mg/ml bovine serum albumin, 25 µg/ml ovotransferrin, 30 nM selenium), penicillin G, and streptomycin (M199/BOTS), with or without additives at 37°C in a 5% CO2 incubator. Cells were fed every 2 d with fresh medium. We refer to these cultures as DCDMLs to distinguish them from related but functionally distinct systems such as central epithelial explants and immortalized lens-derived cell lines (Musil, 2012). Where indicated, DCDMLs were incubated with UO126, PD173074, or dorsomorphin for 45 min at 37°C before addition of growth factors. Anti-BMP antibodies were used at 40 μg/ml and noggin at 0.5 μg/ml.
Plasmids and transient transfection of lens cells
One day after plating, DCDML cultures were transfected in M199 medium without BOTS or antibiotics using Lipofectamine 2000 (Gibco-BRL, Grand Island, NY) following the manufacturer's suggested protocol. Each transfection experiment included both negative (no growth factor) and positive (BMP and/or FGF) controls, with the results presented as fold over the negative control in the same experiment for the same plasmid. Control experiments confirmed that the efficiency of transient transfection of DCDMLs is consistently ∼70% (Boswell et al., 2009). The DCR1-αA-promoter-EGFP reporter (Yang et al., 2006) was a kind gift of Ales Cvekl, Albert Einstein College of Medicine (New York, NY). The BRE-Luc reporter construct (Korchynskyi and ten Dijke, 2002) was provided by Peter ten Dijke (Netherlands Cancer Institute, Amsterdam, Netherlands), and pID1SB-Luc (Nehlin et al., 1997) was a generous gift from Judy Campisi, University of California (Berkeley, CA). The SBE4-Luc (Zawel et al., 1998) and 5′ Del 6 Id1-Luc (Tournay and Benezra, 1996) reporter constructs were provided by Bert Vogelstein (Johns Hopkins University, Baltimore, MD; Addgene [Cambridge, MA] plasmid 16495) and Robert Benezra (Memorial Sloan-Kettering Institute, New York NY; Addgene plasmid 16054), respectively. pEGFP-C2 was from Clontech.
Immunoblot analysis
For CP49, luciferase, and GFP, DCDML cultures were solubilized in lysis buffer as previously described (Le and Musil, 2001a). For all other proteins, cells were solubilized directly in SDS-PAGE sample buffer and boiled. Equal amounts of total protein were transferred to polyvinylidene fluoride membranes, and the blots were probed with primary antibodies. Immunoreactive proteins were detected using secondary antibodies conjugated to either IRDye800 (Rockland Immunochemicals, Limerick, PA) or Alexa Fluor 680 (Molecular Probes, Eugene, OR) and directly quantified using the LI-COR Biosciences Odyssey infrared imaging system (Lincoln, NE) and associated software.
[35S]methionine metabolic labeling
DCDML cultures were labeled at 37°C with [35S]methionine for 4 h in methionine- and serum- free DMEM (Gibco-BRL) and solubilized as previously described (Le and Musil, 1998; 2001a). [35S]methionine incorporation into total cellular protein and into δ-crystallin was quantitated after SDS-PAGE using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA) and IPLab Gel software (Signal Analytics, Vienna, VA).
Immunofluorescence microscopy
DCDMLs grown on glass coverslips were fixed in 2% paraformaldehyde in phosphate-buffered saline and processed for immunocytochemical detection of luciferase (Le and Musil, 1998, 2001b). Immunofluorescence images were captured using a Leica (Wetzlar, Germany) DM LD photomicrography system and Scion Image 1.60 software (Scion, Frederick, MD).
Scrape-loading/dye transfer assay for gap junctional intercellular communication
DCDML cultures grown on laminin-coated coverslips were assessed for gap junction–mediated intercellular coupling as previously described (Le and Musil, 2001b; Boswell et al., 2008a). The distance that Lucifer yellow spreads into the monolayer is directly proportional to the number of coupled cells (Le and Musil, 2001b; Boswell et al., 2008a). In Figure 9A, only the Lucifer yellow channel and only a portion of the right half of the scrape/load wound are shown.
MTT assay for cell proliferation
DCDMLs were plated at 0.7 × 105 cells/well onto laminin-coated, 96-well tissue culture plates. After 16 h, the subconfluent cultures were incubated with fresh M199/BOTS with or without additions for 48 h. Medium was replaced with M199/BOTS containing 0.8 mg/ml MTT and incubated for 2 h at 37°C. Conversion of MTT by living cells into a blue formazan dye was measured at 570 nM according to the manufacture's protocol (kit TOX-1; Sigma-Aldrich) using an automated microtiter plate reader.
qRT-PCR (TaqMan)
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) and the manufacturer's protocol. RNA samples (1 μg) were treated with RNase-free DNase to avoid genomic DNA contamination and then reverse transcribed into cDNA using random primers and the SuperScript III first-strand synthesis system (Invitrogen). Real-time RT-PCR for the target (chicken Id1, FGFR1, FGFR3, BMP7, BMP4) and endogenous reference (GAPDH) genes was carried out in separate tubes in duplicate using the LightCycler 480 (Roche, Indianapolis, IN) sequence detection system and fluorescein amidite–labeled probes and primers. Negative control samples (no template or no enzyme in the reverse transcription reaction) were included. Thermal cycling conditions were as follows: 95°C for 10 min, 50 cycles of 95°C/10 s and 60°C/20 s, and 40°C for 30 s. PCRs contained twofold dilutions of cDNA template, TaqMan Universal PCR Master Mix, and TaqMan Assay Mix (for Id1, FGFR1, FGFR3, BMP7, BMP4, and GAPDH, assay IDs were, respectively, Gg03337774_g1, Gg03340351_m1, Gg03340329_m1, Gg03310499_m1, Gg03348675_m1, and Gg03346982_m1). All data were captured using LCS480 1.5.0.39 software and exported to Excel (Microsoft, Redmond, WA) worksheets for further analysis. Relative amounts of target cDNA (normalized to GAPDH) were calculated by the comparative CT (threshold cycle) method (User Bulletin #2; Applied Biosystems, Carlsbad, CA) and plotted as fold increase compared with untreated (or, in Table 1C, DRB only) samples in the same experiment. Standard curves confirmed that the efficiency of amplification of GAPDH and of target genes was approximately equal (≥0.95).
Acknowledgments
This work was supported by Grants R01 2EY014622 and EY022113 from the National Eye Institute to L.M. We thank P. FitzGerald, B. Druker, P. Stork, P. Rotwein, B. Horton, A. Cvekl, P. ten Dijke, and J. Campisi for generously providing antibodies and other reagents and Janice Paterson for invaluable help with qRT-PCR.
Abbreviations used:
- BMP
bone morphogenetic protein
- BMPR
BMP receptor
- BRE
BMP response element
- DCDML
dissociated cell–derived monolayer culture
- DRB
5,6-dichloro-1-β-d-ribofuranosylbenzimidazole
- ERK
extracellular signal–regulated kinase
- FGF
fibroblast growth factor
- FGFR
FGF receptor
- GJIC
gap junction–mediated intercellular communication
- TGFβ
transforming growth factor β
- TPA
12-O-tetradecanoylphorbal-13-acetate.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E15-02-0117) on May 6, 2015.
REFERENCES
- Bakrania P, Efthymiou M, Klein JC, Salt A, Bunyan DJ, Wyatt A, Ponting CP, Martin A, Williams S, Lindley V, et al. Mutations in BMP4 cause eye, brain, and digit developmental anomalies: overlap between the BMP4 and hedgehog signaling pathways. Am J Hum Genet. 2008;82:304–319. doi: 10.1016/j.ajhg.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo GJ, Mathias RT. Spatial variations in membrane properties in the intact rat lens. Biophys J. 1992;63:518–529. doi: 10.1016/S0006-3495(92)81624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe D, Garcia C, Wang X, Rajagopal R, Feldmeier M, Kim JY, Chytil A, Moses H, Ashery-Padan R, Rauchman M. Contributions by members of the TGFbeta superfamily to lens development. Int J Dev Biol. 2004;48:845–856. doi: 10.1387/ijdb.041869db. [DOI] [PubMed] [Google Scholar]
- Boswell BA, Le AC, Musil LS. Upregulation and maintenance of gap junctional communication in lens cells. Exp Eye Res. 2009;88:919–927. doi: 10.1016/j.exer.2008.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell BA, Lein PJ, Musil LS. Cross-talk between fibroblast growth factor and bone morphogenetic proteins regulates gap junction-mediated intercellular communication in lens cells. Mol Biol Cell. 2008a;19:2631–2641. doi: 10.1091/mbc.E08-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell BA, Overbeek PA, Musil LS. Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev Biol. 2008b;324:202–212. doi: 10.1016/j.ydbio.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Kroog GS. Alterations in receptor expression or agonist concentration change the pathways gastrin-releasing peptide receptor uses to regulate extracellular signal-regulated kinase. Mol Pharmacol. 2004;66:1625–1634. doi: 10.1124/mol.104.001206. [DOI] [PubMed] [Google Scholar]
- Cho JY, Guo C, Torello M, Lunstrum GP, Iwata T, Deng C, Horton WA. Defective lysosomal targeting of activated fibroblast growth factor receptor 3 in achondroplasia. Proc Natl Acad Sci USA. 2004;101:609–614. doi: 10.1073/pnas.2237184100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes LJ, Elliott RM, Reddan JR, Wormstone YM, Wormstone IM. Oligonucleotide microarray analysis of human lens epithelial cells: TGFbeta regulated gene expression. Mol Vis. 2007;13:1181–1197. [PubMed] [Google Scholar]
- de Iongh RU, Chen Y, Kokkinos MI, McAvoy JW. BMP and activin receptor expression in lens development. Mol Vis. 2004;10:566–576. [PubMed] [Google Scholar]
- Dias da Silva MR, Tiffin N, Mima T, Mikawa T, Hyer J. FGF-mediated induction of ciliary body tissue in the chick eye. Dev Biol. 2007;304:272–285. doi: 10.1016/j.ydbio.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson P, Kistler J, Mathias RT. Molecular solutions to mammalian lens transparency. News Physiol Sci. 2001;16:118–123. doi: 10.1152/physiologyonline.2001.16.3.118. [DOI] [PubMed] [Google Scholar]
- Du Y, Xiao Q, Yip HK. Regulation of retinal progenitor cell differentiation by bone morphogenetic protein 4 is mediated by the smad/id cascade. Invest Ophthalmol Vis Sci. 2010;51:3764–3773. doi: 10.1167/iovs.09-4906. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Godin RE, Robertson EJ. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev. 1999;13:1601–1613. doi: 10.1101/gad.13.12.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Faber SC, Dimanlig P, Makarenkova HP, Shirke S, Ko K, Lang RA. Fgf receptor signaling plays a role in lens induction. Development. 2001;128:4425–4438. doi: 10.1242/dev.128.22.4425. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan V, Overbeek PA. Secreted FGFR3, but not FGFR1, inhibits lens fiber differentiation. Development. 2001;128:1617–1627. doi: 10.1242/dev.128.9.1617. [DOI] [PubMed] [Google Scholar]
- Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AN, Kwiatkowski W, Affolter M, Vale WW, Belmonte JC, Choe S. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature. 2002;420:636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem. 1999;274:19838–19845. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- Hung FC, Zhao S, Chen Q, Overbeek PA. Retinal ablation and altered lens differentiation induced by ocular overexpression of BMP7. Vision Res. 2002;42:427–438. doi: 10.1016/s0042-6989(01)00242-5. [DOI] [PubMed] [Google Scholar]
- Isaac A, Cohn MJ, Ashby P, Ataliotis P, Spicer DB, Cooke J, Tickle C. FGF and genes encoding transcription factors in early limb specification. Mech Dev. 2000;93:41–48. doi: 10.1016/s0925-4773(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Jarrin M, Pandit T, Gunhaga L. A balance of FGF and BMP signals regulates cell cycle exit and Equarin expression in lens cells. Mol Biol Cell. 2012;23:3266–3274. doi: 10.1091/mbc.E12-01-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaid A, Neves J, Giraldez F. Id gene regulation and function in the prosensory domains of the chicken inner ear: a link between Bmp signaling and Atoh1. J Neurosci. 2010;30:11426–11434. doi: 10.1523/JNEUROSCI.2570-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T, Imada M, Yanai T, Suda T, Takahashi N, Kamijo R. Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells. 2002;7:949–960. doi: 10.1046/j.1365-2443.2002.00573.x. [DOI] [PubMed] [Google Scholar]
- Knights V, Cook SJ. De-regulated FGF receptors as therapeutic targets in cancer. Pharmacol Ther. 2010;125:105–117. doi: 10.1016/j.pharmthera.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp UR. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- Kovalenko D, Yang X, Nadeau RJ, Harkins LK, Friesel R. Sef inhibits fibroblast growth factor signaling by inhibiting FGFR1 tyrosine phosphorylation and subsequent ERK activation. J Biol Chem. 2003;278:14087–14091. doi: 10.1074/jbc.C200606200. [DOI] [PubMed] [Google Scholar]
- Kozmikova I, Candiani S, Fabian P, Gurska D, Kozmik Z. Essential role of Bmp signaling and its positive feedback loop in the early cell fate evolution of chordates. Dev Biol. 2013;382:538–554. doi: 10.1016/j.ydbio.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- Le AC, Musil LS. Normal differentiation of cultured lens cells after inhibition of gap junction-mediated intercellular communication. Dev Biol. 1998;204:80–96. doi: 10.1006/dbio.1998.9030. [DOI] [PubMed] [Google Scholar]
- Le AC, Musil LS. FGF signaling in chick lens development. Dev Biol. 2001a;233:394–411. doi: 10.1006/dbio.2001.0194. [DOI] [PubMed] [Google Scholar]
- Le AC, Musil LS. A novel role for FGF and extracellular signal-regulated kinase in gap junction-mediated intercellular communication in the lens. J Cell Biol. 2001b;154:197–216. doi: 10.1083/jcb.200101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Tao C, Cai Z, Hertzler-Schaefer K, Collins TN, Wang F, Feng GS, Gotoh N, Zhang X. Frs2a and Shp2 signal independently of Gab to mediate FGF signaling in lens development. J Cell Sci. 2014;127:571–582. doi: 10.1242/jcs.134478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Oparil S, Sun JZ, Thompson JA, Chen YF. Fibroblast growth factor mediates hypoxia-induced endothelin—a receptor expression in lung artery smooth muscle cells. J Appl Physiol. 2003;95:643–651. doi: 10.1152/japplphysiol.00652.2002. [DOI] [PubMed] [Google Scholar]
- Logeart-Avramoglou D, Bourguignon M, Oudina K, Ten Dijke P, Petite H. An assay for the determination of biologically active bone morphogenetic proteins using cells transfected with an inhibitor of differentiation promoter-luciferase construct. Anal Biochem. 2006;349:78–86. doi: 10.1016/j.ab.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Lopez-Rovira T, Chalaux E, Massague J, Rosa JL, Ventura F. Direct binding of Smad1 and Smad4 to two distinct motifs mediates bone morphogenetic protein-specific transcriptional activation of Id1 gene. J Biol Chem. 2002;277:3176–3185. doi: 10.1074/jbc.M106826200. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development. 2001;128:5075–5084. doi: 10.1242/dev.128.24.5075. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW, de Iongh RU. Understanding the role of growth factors in embryonic development: insights from the lens. Philos Trans R Soc Lond B Biol Sci. 2011;366:1204–1218. doi: 10.1098/rstb.2010.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125:3365–3377. doi: 10.1242/dev.125.17.3365. [DOI] [PubMed] [Google Scholar]
- Madakashira BP, Kobrinski DA, Hancher AD, Arneman EC, Wagner BD, Wang F, Shin H, Lovicu FJ, Reneker LW, Robinson ML. Frs2a enhances fibroblast growth factor-mediated survival and differentiation in lens development. Development. 2012;139:4601–4612. doi: 10.1242/dev.081737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier E, von Hofsten J, Nord H, Fernandes M, Paek H, Hébert JM, Gunhaga L. Opposing Fgf and Bmp activities regulate the specification of olfactory sensory and respiratory epithelial cell fates. Development. 2010;137:1601–1611. doi: 10.1242/dev.051219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev. 2003;17:2993–2997. doi: 10.1101/gad.1167003. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiol Rev. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–228. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG, de Iongh RU, Hales AM, Lovicu FJ. Lens development. Eye. 1999;13:425–437. doi: 10.1038/eye.1999.117. [DOI] [PubMed] [Google Scholar]
- Menko AS, Klukas KA, Johnson RG. Chicken embryo lens cultures mimic differentiation in the lens. Dev Biol. 1984;103:129–141. doi: 10.1016/0012-1606(84)90014-9. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Masai I. The lens equator: a platform for molecular machinery that regulates the switch from cell proliferation to differentiation in the vertebrate lens. Dev Growth Differ. 2014;56:387–401. doi: 10.1111/dgd.12128. [DOI] [PubMed] [Google Scholar]
- Monteiro RM, de Sousa Lopes SM, Korchynskyi O, ten Dijke P, Mummery CL. Spatio-temporal activation of Smad1 and Smad5 in vivo: monitoring transcriptional activity of Smad proteins. J Cell Sci. 2004;117:4653–4663. doi: 10.1242/jcs.01337. [DOI] [PubMed] [Google Scholar]
- Musil LS. Primary cultures of embryonic chick lens cells as a model system to study lens gap junctions and fiber cell differentiation. J Membr Biol. 2012;245:357–368. doi: 10.1007/s00232-012-9458-y. [DOI] [PubMed] [Google Scholar]
- Nehlin JO, Hara E, Kuo WL, Collins C, Campisi J. Genomic organization, sequence, and chromosomal localization of the human helix-loop-helix Id1 gene. Biochem Biophys Res Commun. 1997;231:628–634. doi: 10.1006/bbrc.1997.6152. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S, Wood H, Kondoh H, Lovell-Badge R, Episkopou V. Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 1998;12:776–781. doi: 10.1101/gad.12.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswander L, Martin GR. FGF-4 and BMP-2 have opposite effects on limb growth. Nature. 1993;361:68–71. doi: 10.1038/361068a0. [DOI] [PubMed] [Google Scholar]
- Obata H, Kaji Y, Yamada H, Kato M, Tsuru T, Yamashita H. Expression of transforming growth factor-beta superfamily receptors in rat eyes. Acta Ophthalmol Scand. 1999;77:151–156. doi: 10.1034/j.1600-0420.1999.770207.x. [DOI] [PubMed] [Google Scholar]
- Pan Y, Woodbury A, Esko JD, Grobe K, Zhang X. Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development. 2006;133:4933–4944. doi: 10.1242/dev.02679. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Huang J, Dattilo LK, Kaartinen V, Mishina Y, Deng CX, Umans L, Zwijsen A, Roberts AB, Beebe DC. The type I BMP receptors, Bmpr1a and Acvr1, activate multiple signaling pathways to regulate lens formation. Dev Biol. 2009;335:305–316. doi: 10.1016/j.ydbio.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal R, Ishii S, Beebe DC. Intracellular mediators of transforming growth factor beta superfamily signaling localize to endosomes in chicken embryo and mouse lenses in vivo. BMC Cell Biol. 2007;8:25. doi: 10.1186/1471-2121-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold MI, Abe M, Kapadia RM, Liao Z, Naski MC. FGF18 represses noggin expression and is induced by calcineurin. J Biol Chem. 2004;279:38209–38219. doi: 10.1074/jbc.M404855200. [DOI] [PubMed] [Google Scholar]
- Reneker LW, Overbeek PA. Lens-specific expression of PDGF-A alters lens growth and development. Dev Biol. 1996;180:554–565. doi: 10.1006/dbio.1996.0328. [DOI] [PubMed] [Google Scholar]
- Reuss B, von Bohlen und Halbach O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313:139–157. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- Rice R, Thesleff I, Rice DP. Regulation of Twist, Snail, and Id1 is conserved between the developing murine palate and tooth. Dev Dynam. 2005;234:28–35. doi: 10.1002/dvdy.20501. [DOI] [PubMed] [Google Scholar]
- Richardson NA, Chamberlain CG, McAvoy JW. IGF-1 enhancement of FGF-induced lens fiber differentiation in rats of different ages. Invest Ophthalmol Vis Sci. 1993;34:3303–3312. [PubMed] [Google Scholar]
- Richardson NA, McAvoy JW, Chamberlain CG. Age of rats affects response of lens epithelial explants to fibroblast growth factor. Exp Eye Res. 1992;55:649–656. doi: 10.1016/0014-4835(92)90169-s. [DOI] [PubMed] [Google Scholar]
- Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17:726–740. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt-Rosen O, Mosonego-Ornan E, Sadot E, Madar-Shapiro L, Sheinin Y, Ginsberg D, Yayon A. Induction of chondrocyte growth arrest by FGF: transcriptional and cytoskeletal alterations. J Cell Sci. 2002;115:553–562. doi: 10.1242/jcs.115.3.553. [DOI] [PubMed] [Google Scholar]
- Sapkota G, Alarcon C, Spagnoli FM, Brivanlou AH, Massague J. Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol Cell. 2007;25:441–454. doi: 10.1016/j.molcel.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Satow R, Kurisaki A, Chan TC, Hamazaki TS, Asashima M. Dullard promotes degradation and dephosphorylation of BMP receptors and is required for neural induction. Dev Cell. 2006;11:763–774. doi: 10.1016/j.devcel.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Schuler-Metz A, Knöchel S, Kaufmann E, Knöchel W. The homeodomain transcription factor Xvent-2 mediates autocatalytic regulation of BMP-4expression in Xenopus embryos. J Biol Chem. 2000;275:34365–34374. doi: 10.1074/jbc.M003915200. [DOI] [PubMed] [Google Scholar]
- Schulz MW, Chamberlain CG, de Iongh RU, McAvoy JW. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development. 1993;118:117–126. doi: 10.1242/dev.118.1.117. [DOI] [PubMed] [Google Scholar]
- Shin M, Ohte S, Fukuda T, Sasanuma H, Yoneyama K, Kokabu S, Miyamoto A, Tsukamoto S, Hohjoh H, Jimi E, Katagiri T. Identification of a novel bone morphogenetic protein (BMP) inducible transcript, BMP-inducible transcript-1, by utilizing the conserved BMP-responsive elements in the Id genes. J Bone Miner Metab. 2013;31:34–43. doi: 10.1007/s00774-012-0381-1. [DOI] [PubMed] [Google Scholar]
- Sjödal M, Edlund T, Gunhaga L. Time of exposure to BMP signals plays a key role in the specification of the olfactory and lens placodes ex vivo. Dev Cell. 2007;13:141–149. doi: 10.1016/j.devcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Song K, Krause C, Shi S, Patterson M, Suto R, Grgurevic L, Vukicevic S, van Dinther M, Falb D, Ten Dijke P, Alaoui-Ismaili MH. Identification of a key residue mediating bone morphogenetic protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity. J Biol Chem. 2010;285:12169–12180. doi: 10.1074/jbc.M109.087197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MH, Garland DL, Truscott RJ. Movement of cysteine in intact monkey lenses: the major site of entry is the germinative region. Exp Eye Res. 2003;77:245–251. doi: 10.1016/s0014-4835(03)00110-6. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Korchynskyi O, Valdimarsdottir G, Goumans MJ. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211:105–113. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Thut CJ, Rountree RB, Hwa M, Kingsley DM. A large-scale in situ screen provides molecular evidence for the induction of eye anterior segment structures by the developing lens. Dev Biol. 2001;231:63–76. doi: 10.1006/dbio.2000.0140. [DOI] [PubMed] [Google Scholar]
- Tournay O, Benezra R. Transcription of the dominant-negative helix-loop-helix protein Id1 is regulated by a protein complex containing the immediate-early response gene Egr-1. Mol Cell Biol. 1996;16:2418–2430. doi: 10.1128/mcb.16.5.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- Vargas MR, Pehar M, Cassina P, Martínez-Palma L, Thompson JA, Beckman JS, Barbeito L. Fibroblast growth factor-1 induces heme oxygenase-1 via nuclear factor erythroid 2-related factor 2 (Nrf2) in spinal cord astrocytes: consequences for motor neuron survival. J Biol Chem. 2005;280:25571–25579. doi: 10.1074/jbc.M501920200. [DOI] [PubMed] [Google Scholar]
- Walshe J, Mason I. Expression of FGFR1, FGFR2 and FGFR3 during early neural development in the chick embryo. Mech Dev. 2000;90:103–110. doi: 10.1016/s0925-4773(99)00225-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Weitzmann MN, Sangadala S, Hutton WC, Yoon ST. Link protein N-terminal peptide binds to bone morphogenetic protein (BMP) type II receptor and drives matrix protein expression in rabbit intervertebral disc cells. J Biol Chem. 2013;288:28243–28253. doi: 10.1074/jbc.M113.451948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SM, Brunet LJ, Harland RM, Economides AN, Longaker MT. The BMP antagonist noggin regulates cranial suture fusion. Nature. 2003;422:625–629. doi: 10.1038/nature01545. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Dev Biol. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development. 2000;127:2695–2704. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- Whitman M. Smads and early developmental signaling by the TGFbeta superfamily. Genes Dev. 1998;12:2445–2462. doi: 10.1101/gad.12.16.2445. [DOI] [PubMed] [Google Scholar]
- Wijgerde M, Karp S, McMahon J, McMahon AP. Noggin antagonism of BMP4 signaling controls development of the axial skeleton in the mouse. Dev Biol. 2005;286:149–157. doi: 10.1016/j.ydbio.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Wilson I, Edlund T. Neural induction: toward a unifying mechanism. Nat Neurosci. 2001;4:1161–1168. doi: 10.1038/nn747. [DOI] [PubMed] [Google Scholar]
- Wolf L, Gao CS, Gueta K, Xie Q, Chevallier T, Podduturi NR, Sun J, Conte I, Zelenka PS, Ashery-Padan R, et al. Identification and characterization of FGF2-dependent mRNA: microRNA networks during lens fiber cell differentiation. G3 (Bethesda) 2013;3:2239–2255. doi: 10.1534/g3.113.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A, Lamothe B, Lee A, Schlessinger J, Lax I. FRS2 alpha attenuates FGF receptor signaling by Grb2-mediated recruitment of the ubiquitin ligase Cbl. Proc Natl Acad Sci USA. 2002;99:6684–6689. doi: 10.1073/pnas.052138899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian W, Schwertfeger KL, Rosen JM. Distinct roles of fibroblast growth factor receptor 1 and 2 in regulating cell survival and epithelial-mesenchymal transition. Mol Endocrinol. 2007;21:987–1000. doi: 10.1210/me.2006-0518. [DOI] [PubMed] [Google Scholar]
- Xu N, Brodin P, Wei T, Meisgen F, Eidsmo L, Nagy N, Kemeny L, Ståhle M, Sonkoly E, Pivarcsi A. MiR-125b, a microRNA downregulated in psoriasis, modulates keratinocyte proliferation by targeting FGFR2. J Invest Dermatol. 2011;131:1521–1529. doi: 10.1038/jid.2011.55. [DOI] [PubMed] [Google Scholar]
- Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- Yang Y, Stopka T, Golestaneh N, Wang Y, Wu K, Li A, Chauhan BK, Gao CY, Cveklova K, Duncan MK, et al. Regulation of alphaA-crystallin via Pax6, c-Maf, CREB and a broad domain of lens-specific chromatin. EMBO J. 2006;25:2107–2118. doi: 10.1038/sj.emboj.7601114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BS, Pogue R, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. BMPs regulate multiple aspects of growth-plate chondrogenesis through opposing actions on FGF pathways. Development. 2006;133:4667–4678. doi: 10.1242/dev.02680. [DOI] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell. 1998;1:611–617. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- Zhao S, Chen Q, Hung FC, Overbeek PA. BMP signaling is required for development of the ciliary body. Development. 2002;129:4435–4442. doi: 10.1242/dev.129.19.4435. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–288. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberberg L, ten Dijke P, Sakai LY, Rifkin DB. A rapid and sensitive bioassay to measure bone morphogenetic protein activity. BMC Cell Biol. 2007;8:41. doi: 10.1186/1471-2121-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]