Figure 2.
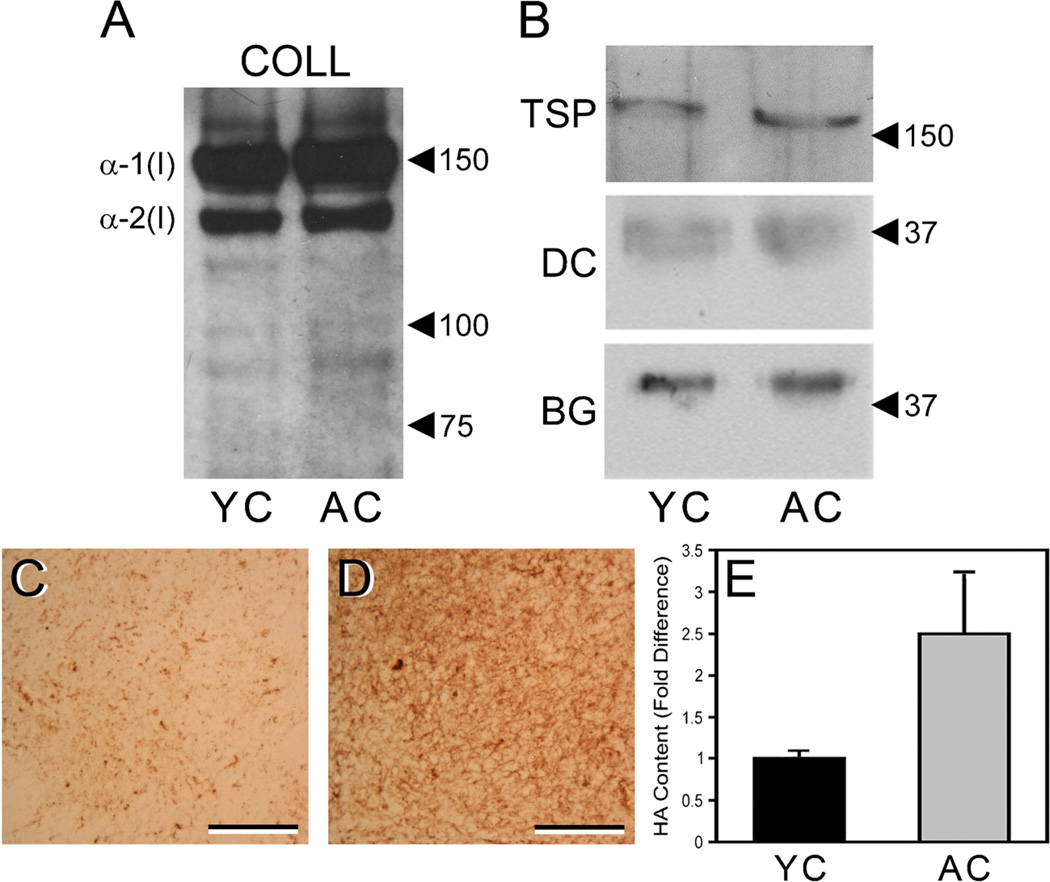
Young and aged collagen extracts have similar content of type I collagen and associated non-collagenous ECM components, but there is increased content of HA in aged collagen. Extracts of young and aged collagen (YC, AC) were resolved by SDS-PAGE under reducing conditions, transferred to nitrocellulose, and stained with antibodies to specific ECM components. Both YC and AC extracts showed strong and similar staining for α-1(I) and α-2(I) chains of type I collagen (blot “COLL”) (A) and weak staining of lower MW bands that likely represent collagen degradation products. TSP-1, decorin, and biglycan were also found in similar quantities in the YC and AC extracts (B) (blots “TSP,” “DC,” and “BG,” respectively). In A and B, positions of MW markers (values are in kDa) are indicated by arrowheads. Sections of gels prepared from YC (C) and AC (D) extracts were stained with HABP to demonstrate total HA content. Note the substantially greater staining (brown color) of the aged gel vs. the young gel. In C and D, scale bars=50 µm. Correspondingly, quantitative ELSA assays of the collagen extracts (E) indicated that the AC extracts (gray bar) contained an average of 2.5-fold more HA than did the YC extracts (black bar), as determined by three separate assays of pooled extracts representing a total of 15 young and 16 aged mice. Average HA content was 8.02±1.6 ng HA per µg aged collagen vs. 3.88±2.4 ng HA per µg young collagen. Values are mean±SEM.