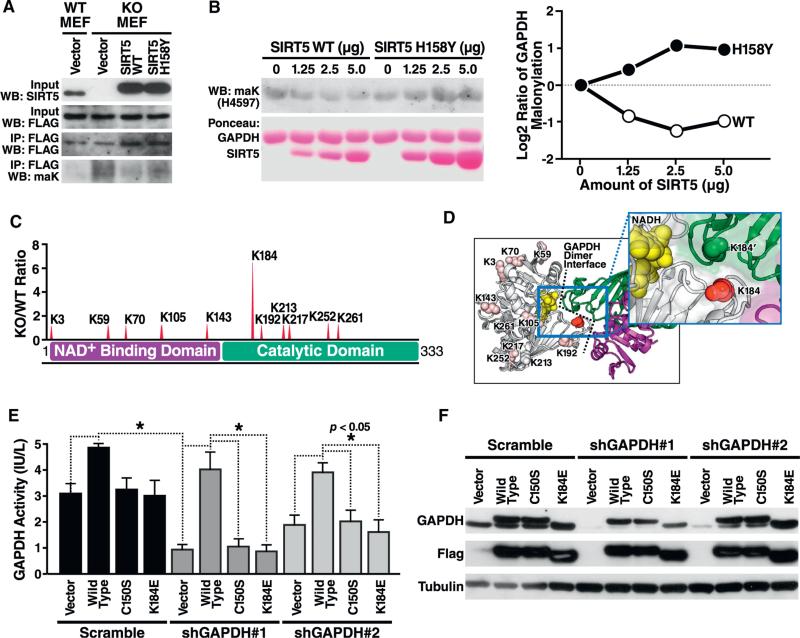
Figure 6. The Major Site of Malonylation in GAPDH by SIRT5 (K184) Is Important for GAPDH Enzymatic Activity.
(A) GAPDH is reversibly de-malonylated by WT but not enzymatically inactive mutant SIRT5 overexpression in cell culture. Flag-tagged GAPDH and SIRT5 were co-overexpressed in WT or SIRT5 KO MEFs, and western blotting against maK was performed following immunoprecipitation by anti-Flag antibody.
(B) GAPDH is reversibly de-malonylated by SIRT5 in vitro. After purifying GAPDH and SIRT5 WT or H158Y enzymatically inactive mutant overexpressed in HEK293T cells, respectively, de-malonylation was performed by adding NAD. MaK level of GAPDH was assayed by western blotting. The quantification of maK levels is shown in the right panel, in which WT but not H158Y SIRT5 effectively demalonylated GAPDH in vitro.
(C) Sites of malonylation in the GAPDH protein with respect to its two functional domains, NAD binding, and catalytic domains. The ratio of malonylation between WT and Sirt5−/− samples are shown above.
(D) Relative malonylation between WT and Sirt5−/− samples is illustrated as a redness color scale, with the most SIRT5-regulated lysine (K184) shown as 100% red.
(E) K184E mutant shows defective GAPDH enzymatic activity. Endogenous human GAPDH was knocked down with two different shRNAs in HEK293T cells after a 4-day selection with puromycin. Expression vector for Flag-tagged mouse GAPDH WT, or mutants C150S (enzymatically inactive) and K184E were overexpressed. GAPDH activity was measured in whole-cell lysates 24 hr after transfection of expression vectors by enzymatic assay.
(F) Knockdown of endogenous GAPDH and expression of WT, C150S, and K184E mutants was confirmed by western blotting using an antiserum against GAPDH or against Flag.