Abstract
The retrotrapezoid/parafacial respiratory group (RTN/pFRG) located ventral to the facial nucleus plays a key role in regulating breathing, especially enhanced expiratory activity during hypercapnic conditions. To clarify the roles of the RTN/pFRG region in evoking coughing, during which reflexive enhanced expiration is produced, and in swallowing, during which the expiratory activity is consistently halted, we recorded extracellular activity from RTN/pFRG neurons during these fictive behaviors in decerebrate, paralyzed, and artificially ventilated guinea pigs. The activity of the majority of recorded respiratory neurons was changed in synchrony with coughing and swallowing. To further evaluate the contribution of RTN/pFRG neurons to these nonrespiratory behaviors, the motor output patterns during breathing, coughing, and swallowing were compared before and after brain stem transection at the caudal margin of RTN/pFRG region. In addition, the effects of transection at its rostral margin were also investigated to evaluate pontine contribution to these behaviors. During respiration, transection at the rostral margin attenuated the postinspiratory activity of the recurrent laryngeal nerve. Meanwhile, the late expiratory activity of the abdominal nerve was abolished after caudal transection. The caudal transection also decreased the amplitude of the coughing-related abdominal nerve discharge but did not abolish the activity. Swallowing could be elicited even after the caudal end transection. These findings raise the prospect that the RTN/pFRG contributes to expiratory regulation during normal respiration, although this region is not an essential element of the neuronal networks involved in coughing and swallowing.
Keywords: retrotrapezoid nucleus, parafacial respiratory group, respiration, coughing, swallowing
the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG) consisting of clusters of respiratory-related neurons located ventral to the facial nucleus, including the RTN which contains CO2 chemosensitive neurons (Mulkey et al. 2004), plays an important role in controlling respiration (Lois et al. 2009; Onimaru and Homma 2003; Pearce et al. 1989; Smith et al. 1989). In particular, the regulation of expiratory activity depends on a homeostatic feedback drive from respiratory-related neurons in the RTN/pFRG, which subsequently achieves an accelerated breathing rate and enhanced expiratory activity caused by abdominal muscle activation under hypercapnic conditions, i.e., active expiration (Abdala et al. 2009; Iizuka and Fregosi 2007; Janczewski and Feldman 2006; Pagliardini et al. 2011; Smith et al. 2007). Enhanced expiration is produced not only during hypercapnic condition but also during various nonrespiratory behaviors including coughing, sneezing, and vocalization (Korpáš and Tomori 1979). Because the same respiratory muscles are used for the generation of breathing and for these nonrespiratory behaviors, it is thought that the brain stem respiratory center also contributes to the induction of these nonrespiratory behaviors (Bianchi and Gestreau 2009). On the other hand, swallowing is one of the nonrespiratory behaviors that uses the same respiratory muscles, but expiratory activity almost completely ceases during swallowing (Jean 2001). Cough is a protective reflex that prevents foreign bodies from entering the airway, via an explosive exhalation caused by powerful abdominal muscle activation; higher ejection pressure is produced by powerful abdominal muscle activation against glottal narrowing and then transient glottal opening causes the explosive expiration (Korpáš and Tomori 1979; Pitts et al. 2013). Jakuš et al. (2008) noted that c-fos immunoreactivity is observed in the RTN/pFRG after exposure of the tracheobronchial mucosa to repetitive stimuli that evoke coughing. These findings raise the possibility that the RTN/pFRG also regulates the coughing-related enhanced expiration as an analogy to active expiration. On the other hand, swallow is a spatiotemporally coordinated movement consisting of sequential pharyngeal contraction and laryngeal closure accompanied by the cessation of abdominal activity. This highly patterned activity, during which fundamental respiratory patterns are altered, is provided by the brain stem pattern generator, which overlaps with the respiratory neuronal networks including the RTN/pFRG (Jean 2001). Indeed, Kessler and Jean (1986) have demonstrated that chemical stimulation of the brain stem adjacent to this area decreases the occurrence of swallowing. However, the roles of the RTN/pFRG in these nonrespiratory behaviors have not been well understood. We thus hypothesized that the RTN/pFRG neurons may change their activity during coughing and swallowing as well as respiration in a type-specific manner, to contribute to the regulation of these behaviors. We first monitored extracellular activity of RTN/pFRG neurons during respiration, coughing, and swallowing and assessed whether the neuronal activity was modulated in synchrony with these behaviors in decerebrate, paralyzed guinea pigs. We additionally hypothesized that if the activities of these neurons, including the expiratory neurons that can control active expiration, are in synchrony with cough-related nerve activity, the removal of this region should attenuate the cough reflex and in particular reduce the cough-related abdominal activity. We assumed that if the activity of these neurons is altered during swallowing, the motor activity during swallowing could be influenced by their removal (Abdala et al. 2009). We therefore examined the effect of transverse brain stem transection at the rostral- and caudal-most levels of the RTN/pFRG on these behaviors.
MATERIALS AND METHODS
All experimental procedures conformed to the Physiological Society of Japan Principles for the Care and Use of Animals and were approved by the University Committee for the Use of Animals in Research.
General Surgical Procedures
Experiments were performed on 37 purpose-bred adult male guinea pigs (Hartley, Shimizu Laboratory Supplies, Kyoto, Japan) weighing 500–800 g. Animals were anesthetized with isoflurane (4% for induction, 1.0–2.0% for maintenance) vaporized in 100% O2. The level of anesthesia was titrated such that the mean blood pressure, which was monitored using a catheter inserted into the common carotid artery, was maintained at <100 mmHg. Sufficient anesthesia was given to prevent spontaneous and reflexive movements. The trachea was intubated, and a cannula was placed in a femoral vein for drug administration. Dexamethasone (1 mg/kg) and atropine (0.1 mg/kg) were injected intramuscularly to minimize brain edema and to decrease airway secretions, respectively. Rectal temperature was maintained at 37–38°C using a DC-powered heating pad. Bipolar silver cuff electrodes were placed around the L1 abdominal nerve (ABD) and phrenic nerve (PHR) for recording, and the superior laryngeal nerve (SLN) and facial nerve for stimulation, and the recurrent laryngeal nerve (RLN) for both recording and stimulation on both sides, and covered with Vaseline and mineral oil.
The animals were placed in a stereotaxic frame and were decerebrated at the precollicular level. An occipital craniotomy was then performed to expose the dorsal aspect of the brain stem. The caudal portion of the cerebellum was gently retracted and partially aspirated to visualize the obex and the rostral part of the medulla. After the surgery was completed, anesthesia was discontinued. Subsequently, animals were paralyzed using intravenous injections of vecuronium bromide (Fuji Pharma, Tokyo, Japan; initial injection of 0.3 mg/kg, maintained by hourly injections of 0.15 mg/kg) and artificially ventilated with room air. A bilateral pneumothorax was performed to reduce ventilation-related brain movements. End-tidal CO2 was monitored using an end-tidal CO2 analyzer (Capstar-100 CO2 Analyzer, CWE, Ardmore, PA). Tidal volume and ventilation frequency were adjusted to maintain end-tidal CO2 at 3–5% (i.e., normal conditions) throughout the experimental period, except during recordings. The optimal end-tidal CO2 level at which “active expiration” was produced was determined before a series of recording sessions was started. The ventilator setting was kept constant during a series of recording sessions. The mean blood pressure was kept above 80 mmHg using an intravenous infusion of epinephrine in saline at the rate of 0.005–0.01 mg·kg−1·min−1, if hypotension occurred. At the end of the experiment, animals were perfused transcardially with 300 ml of physiological saline followed by 300 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brain stem was removed and postfixed in the same solution.
Recording Procedures
Penetrations of the electrode for recording were conducted 3–5.5 mm rostral to the obex and 1.5–3.0 mm lateral to the midline, using a glass micropipette filled with 3 M KCl (tip impedance 5–10 MΩ). Activity recorded extracellularly by the microelectrode was amplified (MEZ-8301, Nihon Kohden, Tokyo, Japan) and sampled at 15,000 Hz using a Power 1401 mk 2 data collection system and Spike 2 version 6 software (Cambridge Electronic Design, Cambridge, UK). Activity of each nerve recorded was amplified by a factor of 10,000, filtered with a band pass of 100-10,000 Hz, integrated with a 1-ms time constant, full-wave rectified, and sampled at 2,000 Hz.
Since the RTN/pFRG region is positioned ventral to the facial nucleus (Connelly et al. 1989; Onimaru and Homma 2003), we used antidromic field potentials of facial motoneurons as a physiological landmark for identification of RTN/pFRG neurons. To identify the location of the facial nucleus, we inserted the electrode with a 0.1 mm step in the rostrocaudal and mediolateral direction and identified the antidromic field potentials evoked by the stimulation of the facial motor nerve at the intensity of 0.08–2 mA. When the amplitude of the field potential decreased to <10% of the maximal value, we considered that the electrode was outside of the facial nucleus. Collision testing was performed in some neurons, whose activities were recorded in the area where the antidromic evoked potential induced by the facial nerve stimulation was simultaneously recorded.
We recorded spontaneous activity along with the phrenic nerve discharges to determine whether the neuron in this area had respiratory-related activity. Subsequently, we recorded the neuronal activity during fictive coughing and swallowing. Fictive coughing was evoked by stimulating the afferent fibers of the RLN electrically (pulse duration, 0.2 ms; frequency, 10–20 Hz, intensity 40–80 μA), or by irritating the tracheal mucosa with a silicon tube through the tracheostoma, and identified by a series of neural activities consisting of the preceding phrenic nerve discharge followed by the RLN and abdominal nerve burst, as described elsewhere (Bolser 1991; Grélot and Milano 1991; Shiba et al. 1999; Sugiyama et al. 2014).
Fictive swallowing was identified by bursting activity of the RLN, which was elicited by electrical stimulation of the SLN (pulse duration, 0.2 ms; frequency, 10–20 Hz, intensity, 50–80 μA) (Nishino et al. 1985; Sugiyama et al. 2011, 2014). The activity of the hypoglossal nerve was not recorded in the present study. Some recording sites were marked by electrolytic lesions through the recording electrode (a constant current of 12 μA for 30 min). Spike detection and sorting feature of the Spike2 software were used such that the accuracy of detection of each unit activity was secured. We calculated the maximum firing frequency of respiratory-related RTN/pFRG neurons in each phase by averaging the occurrence of spikes within 0.2 s during which the instantaneous frequency reached maximum. We then compared the peak firing rate of each neuron during control respiration with that during coughing and swallowing using a Wilcoxon signed-rank test with a theoretical median of 1. The control trial rate data were taken from cycles just prior to the stimulation that induced coughing or swallowing. When continuous infusion of epinephrine was needed, the data were discarded because of the possible epinephrine-induced influence on respiratory regulation during the nonrespiratory behaviors as well as during respiration (Arata et al. 1998; Viemari 2008; Yamanishi et al. 2010).
Transection Procedures
The discharge patterns in the abdominal and RLNs during breathing, coughing, and swallowing were compared between before and after brain stem transection at the levels of the rostral or caudal margin of the RTN/pFRG region. To facilitate the late expiratory activity recorded on the abdominal nerve, end-tidal CO2 levels were elevated above 5% during data acquisition in transection experiments. Because the rostrocaudal levels of the rostral and caudal margins of the RTN/pFRG region correspond to the respective margins of the facial nucleus as mentioned above, these levels were determined by recording the antidromic field potentials evoked by stimulation of the facial nerve. After identification of the rostral and caudal margins, the recording electrodes were positioned along with axial plane. Transections were made alongside an electrode initially at the rostralmost level of the facial nucleus using a razor blade mounted on a manipulator arm placed on the stereotaxic frame. After stable breathing resumed (typically requiring several minutes), we recorded the activities of these nerves during respiration. Then, we evoked coughing and swallowing to compare the activity pattern of each nerve between before and after transection. Additional transection at the level of the caudalmost margin of the facial nucleus was made, and neural recording sessions were conducted in the same manner. Only the caudalmost transection was made in some animals. We analyzed the changes in the nerve activities during respiration, coughing, and swallowing due to the brain stem transections. The amplitude of nerve activity was defined as the difference between the baseline and peak height of the integrated neurogram. The baseline activities were defined as the average heights of the neruograms of the phrenic nerve during the expiratory phase, the RLN during 0.5-s windows just before the end of expiration, and the abdominal nerve during the inspiratory phase, since the phrenic, laryngeal, and abdominal muscle motoneurons are not typically activated during expiration, the end of expiration, and inspiration, respectively (Shiba et al. 1999). The amplitudes of the phrenic, RLN, and abdominal nerve activities after brain stem transections were normalized as a percentage of the amplitudes relative to those before transection. We calculated the respiratory rate, the duration of expiration and inspiration, and the changes in the burst amplitudes of the phrenic, RLN, and abdominal nerves due to the transections during respiration. The peak amplitudes of the phrenic, RLN, and abdominal nerve activities during respiration were measured during the inspiratory phase, a 1-s duration just after the end of inspiration, and a 1-s duration at the end of the expiration, respectively. We also measured the duration (e.g., duration of phrenic activity, duration of initial expiratory burst of RLN just after cough-related inspiration, and duration of abdominal nerve burst) and the amplitude of alterations of cough-related activities of these nerves. The maximal amplitudes of the phrenic nerve activity, the initial burst of the cough-induced RLN activity, and the abdominal nerve activity were calculated. In addition, we measured the duration and changes in the amplitude of the swallow-induced RLN activity. The values were averaged for three trials.
Histological Procedures and Data Analyses
Transverse tissue sections of the brain stem of animals, for which unit recordings and lesioning were performed, were made using a freezing microtome at 50 μm, and were stained with neutral red. Meanwhile, the brain stem of animals used in the transection experiments was cut sagittally at 100 μm, and were stained using cresyl violet. As such, we confirmed that the brain stem was precisely transected at the rostral- and candalmost levels of the facial nucleus. Photographs of brain stem sections were captured using a CCD digital camera (DP21, Olympus) mounted on an upright microscope (BX51, Olympus, Tokyo, Japan) and were assembled using PTGui-Pro photostitching software (New House Internet Services BV, The Netherlands). Adobe Illustrator software (Adobe systems, San Jose, CA) was used for drawing these sections. Recording sites were reconstructed on these drawings with reference to the locations of electrical lesions, the relative positions of electrode tracks, and microelectrode depths. Statistical analyses were performed using Prism 5 software (Graph-Pad Software, San Diego, CA). The data were analyzed using the nonparametric one-way ANOVA with Dunn's multiple comparison test after the Kruskal-Wallis test. Pooled data are presented as means ± 1 SE. A P value of <0.05 was considered significant.
RESULTS
Activity of the Respiratory-Related Neurons in the RTN/pFRG During Coughing and Swallowing
Extracellular neuronal activity was recorded from 109 RTN/pFRG cells in 25 animals, which consisted of 29 expiratory, 55 inspiratory, and 25 phase-spanning neurons. The expiratory neurons were subdivided into the following two types based on their firing patterns: augmenting (E-aug) (n = 18) and decrementing (E-dec) (n = 11) firing patterns. All 55 recorded inspiratory neurons displayed an augmenting discharge pattern (I-aug). The phase-spanning neurons were subdivided into the inspiratory-expiratory (IE) (n = 5) and expiratory-inspiratory (EI) (n = 20) neurons. Maximal firing frequencies of these neurons are listed in Table 1. Of all the neurons recorded, 31 (∼30%) neurons were tested for collision by stimulating the facial nerve. However, the collision did not occur in these neurons.
Table 1.
Maximal firing frequency and activities of the respiratory-related neurons in the retrotrapezoid/parafacial respiratory group
| Maximal Firing Frequency, | Coughing |
Swallowing |
||||
|---|---|---|---|---|---|---|
| Active |
Silent |
Active | Silent | |||
| Neuron type | spikes/s | C1 phase | C2 phase | Expiratory phase | ||
| E-aug (n = 18) | 34.4 ± 5.2 | 82.9 (1) | 94.4 ± 20.3* (14) | (3) | 28.4 ± 15.1 (5) | (13) |
| E-dec (n = 11) | 20.8 ± 2.3 | 53.5 ± 19.7 (7) | 24.6 ± 9.2 (2) | (2) | 14.9 ± 2.3 (9) | (2) |
| I-aug (n = 55) | 47.1 ± 3.3 | (13) | (15) | (27) | (0) | (55) |
| IE (n = 5) | 24.3 ± 3.4 | 40.4 ± 16.4 (3) | 40.3 ± 12.9 (2) | (0) | 73.5 ± 42.7 (3) | (2) |
| EI (n = 20) | 22.5 ± 3.6 | 40.6 ± 10.3 (9) | 55.2 ± 9.6* (8) | (3) | 23.6 ± 4.5 (13) | (7) |
| Total (n = 109) | (33) | (41) | (35) | (30) | (79) | |
Data are presented as means ± SE. Regarding the cough-related activity, the values in parentheses indicate the number of neurons whose peak firing rates were in phase with C1 and C2 during coughing, and those that did not discharge throughout the expiratory phase. For I-aug neurons, the term “active neurons” indicates neurons that discharged during both the inspiratory and expiratory phases, while “silent neurons” designates neurons that fired only during the inspiratory phase. Meanwhile, the number of neurons that were active and silent during swallowing are designated in parentheses. For expiratory and phase-spanning neurons, the maximal firing rates during each phase of coughing or during swallowing are indicated. C1 phase, the period during which the recurrent laryngeal nerve (RLN) strongly discharged just after the cough-related inspiration; C2 phase, the duration of the transient attenuation of the RLN activity during the expiratory phase of coughing.
Significant deference from control respiration. See Glossary for additional abbreviations.
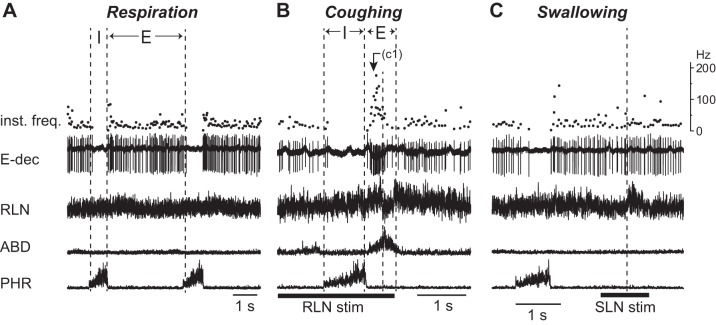
The RLN exhibited a bimodal activity during the expiratory phase of coughing. The RLN began to burst at the transition from the inspiratory to expiratory phase (C1 phase). After this bursting activity, the RLN activity was transiently decreased (C2 phase) and then it burst again (C3 phase). As indicated in Table 1, most (15/18) of the E-aug neurons were activated during the expiratory phase of coughing, all but one of which fired prominently during the C2 phase (Fig. 1A).Their firing frequency during the C2 phase was significantly higher than that of control expiration (P < 0.01) (Table 1). During swallowing, approximately three-quarters of the E-aug neurons (13 of 18) ceased firing (Fig. 1B), and the remaining one-quarter were activated (5 of 18) (Fig. 1C). The firing rate of the swallow-active E-aug neurons during swallowing did not show a significant increase compared with that during control respiration.
Fig. 1.
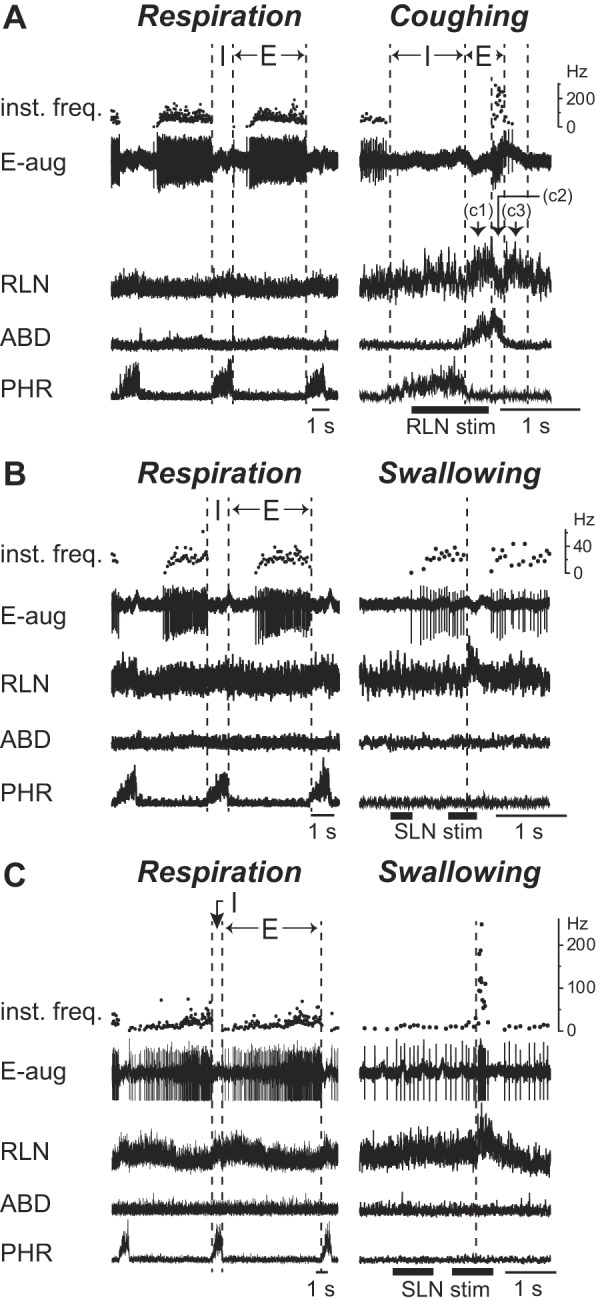
Activity of the E-aug neurons during respiration, coughing, and swallowing. Activity of the expiratory neurons with an augmenting firing pattern (E-aug) during control respiration (left panel) and airway defensive reflexes (right panel) including coughing (A) and swallowing (B and C). The cough-related recurrent laryngeal nerve (RLN) activity shows a triphasic discharge pattern; the initial burst occurs just after the inspiratory-expiratory phase transition (C1 phase), with a subsequent decrease in activity (C2 phase) followed by a reactivation (C3 phase). A: activity of a neuron that fired during the C2 phase. In A, electrical stimulation of the RLN was delivered to evoke fictive coughing. B and C: activity of neurons whose firing was eliminated (B) and those with increased firing (C) during swallowing elicited by electrical stimulation of the superior laryngeal nerve (SLN). In C, the second trace was truncated because of saturation in spike amplification. Dashed vertical lines in the right panels in B and C indicate the onset of the swallow-related RLN burst. ABD, abdominal nerve; PHR, phrenic nerve. See Glossary for additional abbreviations.
The majority of E-dec neurons (9 of 11) were activated during coughing, in which 7 and 2 cells were active during the C1 (Fig. 2B) and C2 phases, respectively; the firing frequency during coughing was not significantly higher than that of control expiration. Most (9 of 11) of the E-dec neurons fired during the swallow-related RLN burst (Fig. 2C); the frequency was not significantly different from that during control respiration.
Fig. 2.
Activity of the E-dec neurons during respiration, coughing, and swallowing. Activity of an expiratory neuron with a decrementing firing pattern (E-dec) during control respiration (A), coughing (B), and swallowing (C). This neuron strongly fired during the C1 phase of coughing (B), whereas the neuron was activated during the swallow-related RLN burst (C). See Glossary for additional abbreviations.
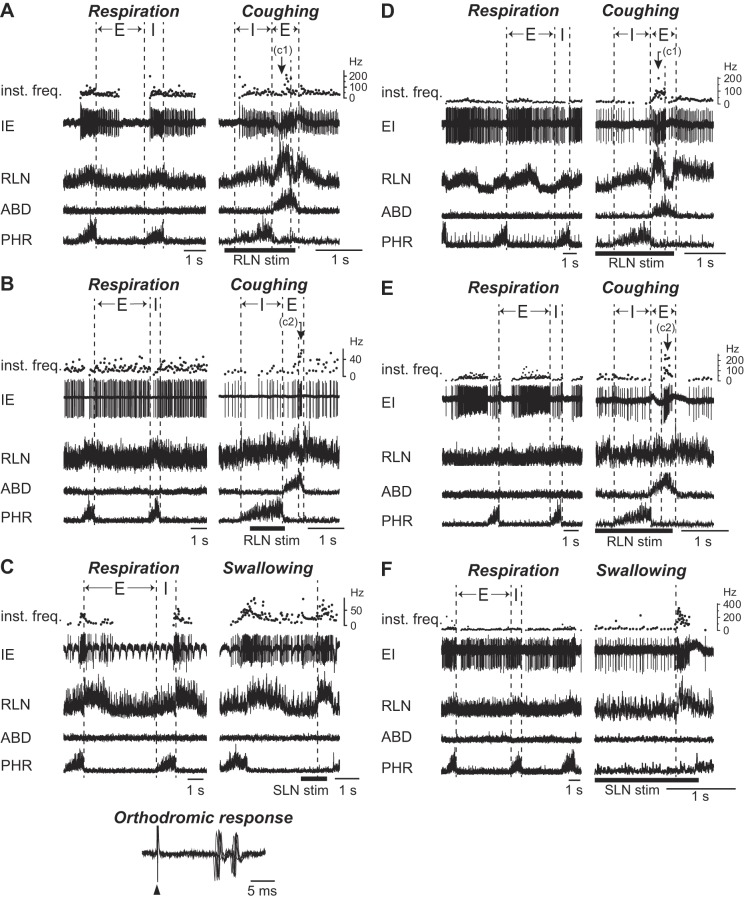
During coughing, about half (27 of 55) of the I-aug neurons were activated only during the inspiratory phase (Fig. 3A). Meanwhile, the remaining I-aug neurons exhibited biphasic activity during coughing, which consisted of augmented inspiratory activity followed by the expiratory discharge with a relatively higher activity in the C1 (Fig. 3B) or C2 (Fig. 3C) phase. During swallowing, the firing of all I-aug neurons was ceased.
Fig. 3.
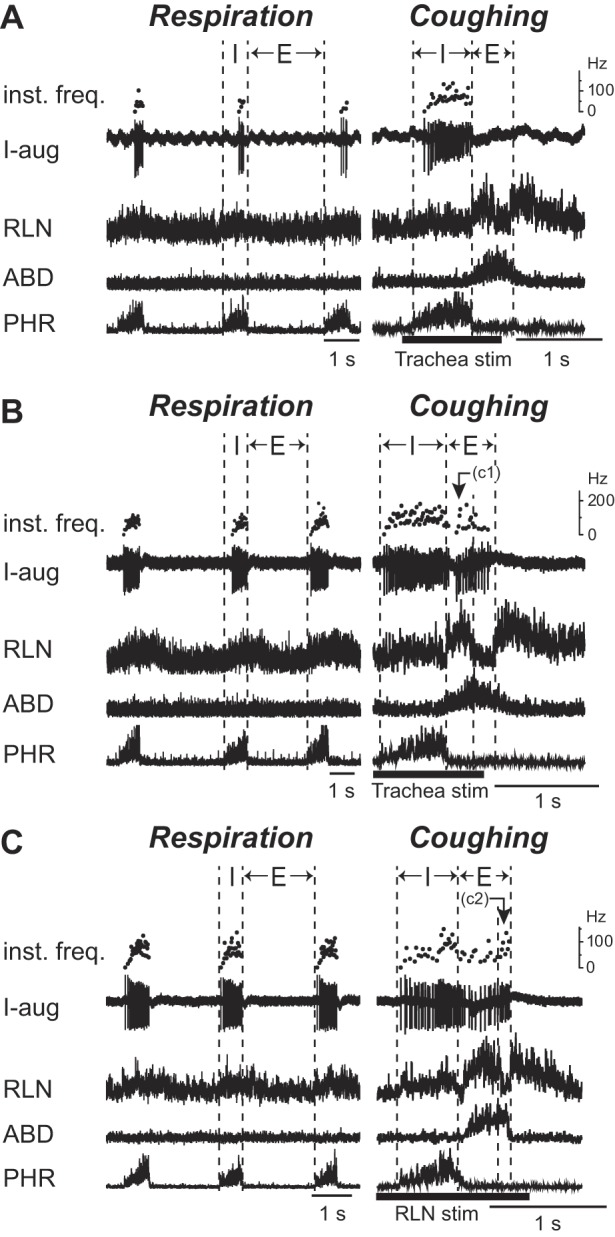
Firing patterns of the I-aug neurons during respiration and coughing. Augmenting firing pattern of inspiratory neurons (I-aug) during respiration (left panel) and coughing (right panel). A: activity of a neuron that exhibited the same firing pattern during coughing as that during breathing. In A, the unit activity during the inspiratory phase of coughing increased compared with that during respiration, with the phrenic nerve discharge enhanced during coughing. B and C: activity of the neurons which fired throughout the coughing period, showing the predominant activity during the C1 (B) or C2 (C) phase in addition to the inspiratory discharge. See Glossary for additional abbreviations.
Most (22 of 25) of the phase-spanning neurons were activated during the expiratory phase of coughing. About one-half of them fired predominantly during the C1 phase (3 of 5 IE, and 9 of 20 EI neurons) (Figs. 4, A and D), while the other half fired predominantly during the C2 phase (2 of 5 IE, and 8 of 20 EI neurons) (Fig. 4, B and E). In the latter EI neurons, the firing frequency during the C2 phase was significantly higher than that of control respiration (P < 0.05). More than half of the phase-spanning neurons discharged during swallowing (Fig. 4, C and F). Only one neuron, represented in Fig. 4C, was orthodromically excited to electrical stimulation of the ipsilateral SLN. There was no significant difference between the firing rates of these neurons during swallowing and those during control respiration. Meanwhile, the comparison across the neuron types during each phase of coughing and swallowing did not show any significant differences.
Fig. 4.
Firing patterns of the phase-spanning neurons during respiration, coughing, and swallowing. Examples of firing patterns of the inspiratory-expiratory (IE) (A–C) and the expiratory-inspiratory (EI) (D–F) phase-spanning neurons during coughing (A, B, D, and E) and swallowing (C, F). A and B: activity of the neurons whose peak firing rate occurred during the C1 (A) and C2 phase (B) of coughing. C: activity of a neuron which fired during swallowing. In C, despite the responses of the neuron to orthodromic activation elicited by the SLN stimuli, as indicated in the bottom panel in C (five superimposed sweeps), the more robust activation was observed during the swallow-related RLN burst. Thus the activity of this neuron was deemed to be modulated with the RLN burst. The neurons in D and E exhibited strong activity during the C1 (D) and C2 phase (E) of coughing. A neuron in F was activated during swallowing. The filled triangle indicates the SLN stimulus. The second trace in C shows the temporal fluctuations in spike amplitude responsible for the pulsations of the brain stem. See Glossary for additional abbreviations.
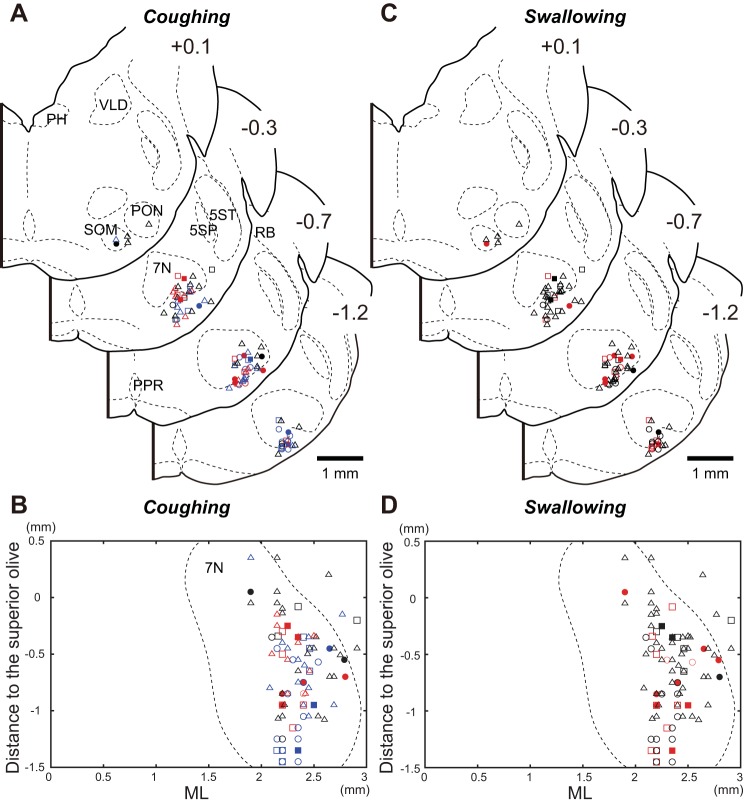
Figure 5 illustrates the locations of a total of 93 neurons in the RTN/pFRG whose activity was analyzed during coughing and swallowing. The symbols of different shapes indicate the 16 E-aug units, 8 E-dec units, 48 I-aug units, 4 IE units, and 17 EI units. These neurons were located 1.9–2.91 mm (average of 2.3 ± 0.02) from the midline, 0.09–1.0 mm (average of 0.4 ± 0.02) from the ventral surface, and −1.45 to 0.35 mm (average of −0.66 ± 0.04) anterior to the most caudal portion of the superior olive. The E-aug neurons were significantly distributed more caudally than the I-aug neurons (P < 0.01). Other types of neurons were intermingled in the RTN/pFRG. In the area ventromedial to the facial nucleus presumably corresponding to the RTN (Connelly et al. 1990) and its vicinity, where CO2-chemosensitive neurons, such as the Phox2b and the vesicular glutamate transporter 2 immunoreactive neurons are located (Guyenet 2008; Mulkey et al. 2004), 32 respiratory neurons were recorded (11 E-aug, 1 E-dec, 13 I-aug, 1 IE, and 6 EI). A total of about 60% of the E-aug neurons was found to be located in this portion of the RTN/pFRG, showing a significantly higher distribution compared with the other portion (Fisher's exact test, P < 0.01).
Fig. 5.
Locations of neurons in the RTN/pFRG with cough- and swallow-related activity. Locations of respiratory-related neurons in the RTN/pFRG responded to the stimulation which evoked coughing (A and B) and swallowing (C and D). Data were obtained from 16 of 25 animals, in which the histologic verification was performed. Cellular locations are plotted on transverse (A and C) and horizontal (B and D) sections. Symbols with different shapes are used to designate the type of neurons with respiratory-related activity. Open circles, filled circles, open triangles, filled squares, and open squares indicate E-aug, E-dec, I-aug, IE, and EI neurons, respectively. In A and B, the neurons which were strongly activated during the C1 and C2 phase of coughing are designated by red and blue symbols, respectively, whereas the neurons that were silent during the expiratory phase of coughing are shown as black symbols. On the other hand, regarding the responses of neurons during swallowing depicted in C and D, red symbols denote neurons that were activated during the period of the swallow-related RLN activity, while black symbols denote neurons which became silent. Numbers at the upper right corner of the transverse section show the rostrocaudal levels (in mm) relative to the position of the caudal pole of the superior olive. See Glossary for additional abbreviations.
Influences of Brain Stem Transections on Motor Nerve Activities During Respiration, Coughing, and Swallowing
Discharge patterns of the laryngeal, abdominal, and phrenic motor nerves during respiration, coughing, and swallowing were recorded before and after transection at the rostral- or caudalmost level of the RTN/pFRG region (Figs. 6 and 7). Of 12 animals, 10 were tested for the effect of removal of the pons by brain stem transection at the rostralmost level of the RTN/pFRG region. After the test of rostral-end transection, the influences of the transection along the caudalmost border were further analyzed in 8 animals. In the other 2 animals, only caudal level transection was examined. The end-tidal CO2 level at which active expiration was induced was 5.5–7%.
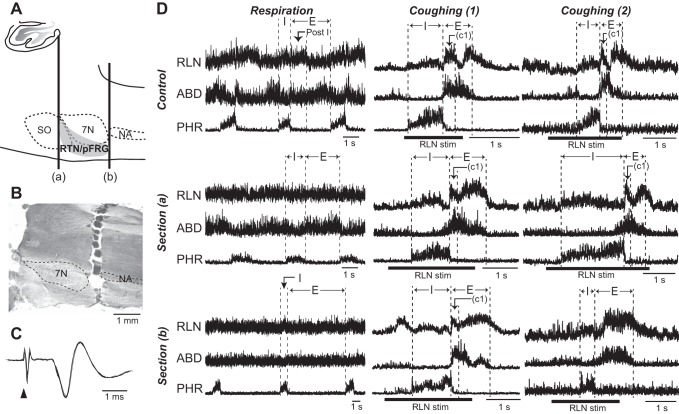
Fig. 6.
Influence of brain stem sectioning on respiratory- and cough-related nerve activities. Changes in the neural motor patterns during respiration before and after transection of the rostral- and caudal-most margins of the RTN/pFRG. A: schematic illustration of the parasagittal section of the brain stem showing the levels of the transverse transection, as indicated by vertical thick lines (a, b). B: a photograph of a parasagittal section of the brain stem. In B, the brain stem tissue is completely separated at the rostral margin of the RTN/pFRG. C: the antidromic field potential evoked by stimulation of the facial motor nerve (5 spikes superimposed). In C, filled triangle indicates electrical stimulation of the facial nerve. D: the motor nerve activities during control respiration (left column) and coughing (middle and right columns). The cough-related activity of the RLN, abdominal, and phrenic nerves whose discharge patterns were preserved and altered after transection at the caudalmost margin of the RTN/pFRG are displayed in the middle [Coughing (1)] and right [Coughing (2)] columns, respectively. The effects of transection at the rostralmost level of the RTN/pFRG on each nerve activity are depicted in the second row [Section (a)], whereas changes in discharge patterns of each nerve after transection of the caudalmost level are shown in the bottom row [Section (b)]. During control respiration, the postinspiratory activity of the RLN and late expiratory activity of the abdominal nerve were observed during hypercapnic ventilation (5.5–7% in end tidal CO2), as indicated in the upper left panel in D. This RLN activity during the postinspiratory phase (Post-I) was eliminated due to the effect of transection of the rostralmost portion, while the late abdominal burst was attenuated by the caudalmost transection. The cough-related RLN and ABD burst preceding phrenic nerve activity still remained subsequent to the rostralmost transection. The cough-related abdominal activity started before termination of the phrenic nerve activity, in the middle right column. After the caudal level transection, the cough-related activity of the RLN during the C1 phase was maintained (middle column), while the RLN burst during the C1 phase was attenuated (right column). See Glossary for additional abbreviations.
Fig. 7.
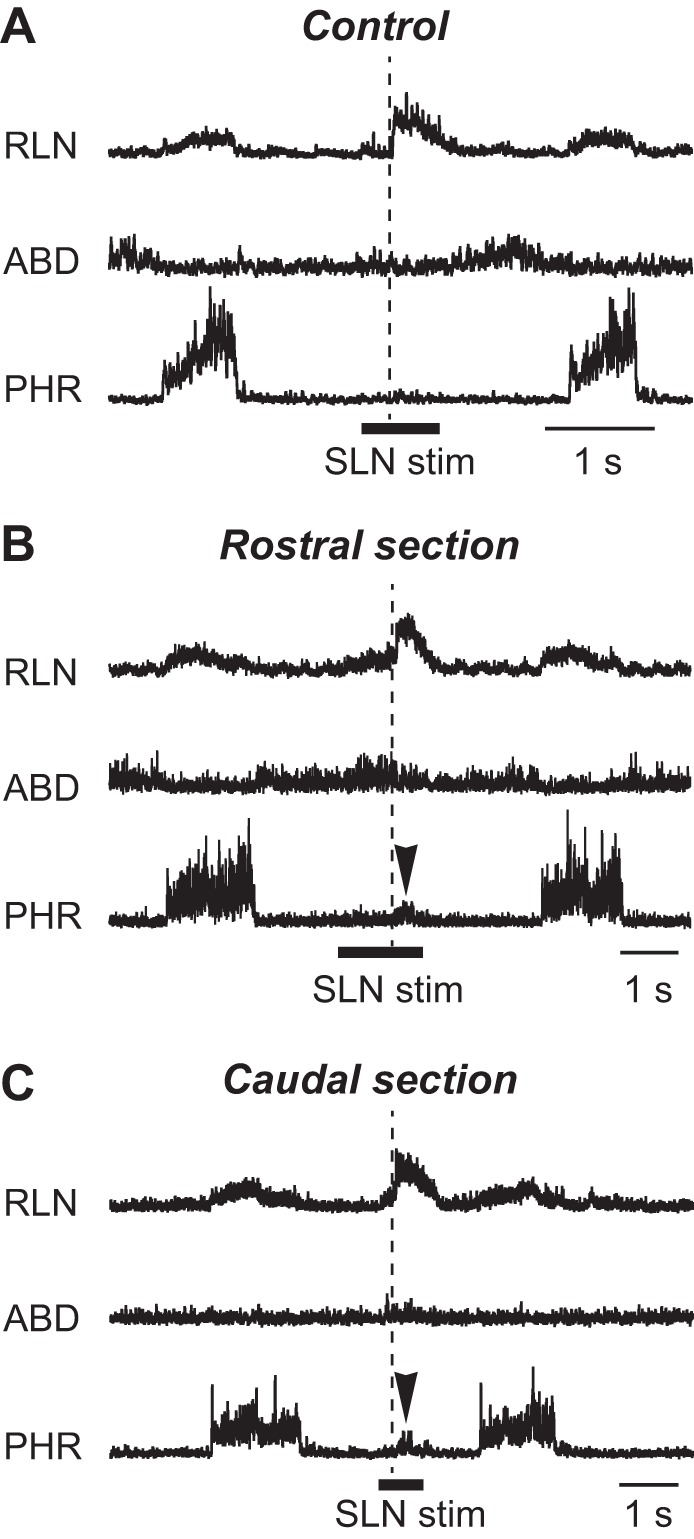
Swallow-related motor nerve activities before and after brain stem sectioning. Transection influences on the activities of laryngeal, abdominal, and phrenic motor nerves during swallowing before (A) and after transection of the brain stem at the level of the rostral- (B) and caudal-most margins (C) of the RTN/pFRG. The swallow-related RLN burst, which can be elicited by stimulation of the SLN afferent fibers, was induced even after either rostral- or caudal-most transection. Arrowhead indicates “Swallow-breath.” See Glossary for additional abbreviations.
The respiratory rates before transection, after rostral section, and after caudal section were 15.7 ± 1.0, 10.5 ± 1.4, and 6.27 ± 0.9 breaths/min, respectively. The durations of the inspiratory phase of respiration before, after rostral, and after caudal transection were 0.77 ± 0.1, 2.40 ± 0.4, and 1.54 ± 0.6, while the expiratory durations were 3.24 ± 0.2, 4.31 ± 0.6, and 11.9 ± 2.6 s, respectively. The transection at the rostralmost level of the RTN/pFRG induced a significant decrease in respiratory rates, and the caudalmost transection exhibited a further decrease (P < 0.01). The expiratory duration was significantly prolonged by the caudalmost transection (P < 0.01), while the inspiratory duration showed no significant difference as a result of the transections. The percentages of amplitudes of the phrenic nerve activity after rostralmost and caudalmost transection relative to those before transection were 79.7 ± 4.7 and 74.7 ± 6.2, whereas the percentages of amplitudes of the RLN activity were 57.3 ± 3.1 and 56.3 ± 2.7, respectively. The amplitudes of the abdominal nerve activity were decreased to 93.8 ± 4.1 and 15.6 ± 2.4% of control levels by rostral- and caudal-level sectioning. The activity of the phrenic nerve and RLN was significantly decreased by the rostralmost sectioning, while the abdominal nerve activity showed a significant decrease after the transection at the caudalmost level of the RTN/pFRG (P < 0.01). As such, during respiration, the rostral-level sectioning attenuated the postinspiratory activity of the RLN, with its inspiratory activity decreased in all tested animals (Fig. 6D). Meanwhile, the enhanced activity of the abdominal nerve in the late expiratory phase induced by hypercapnic ventilation was preserved after the rostral level section, but was abolished after the caudal level section in all tested animals (Fig. 6D). The abdominal nerve activity was not enhanced by an increase of CO2 concentration of up to 10% after caudal transection.
In all animals tested, a series of cough-related discharge patterns of the RLN, abdominal, and phrenic nerves was preserved after the rostralmost transection (Fig. 6D). The caudal-level sectioning preserved the cough-related activity pattern in the RLN in 3 animals (middle column in Fig. 6D) but altered its bimodal discharge pattern to a single peak one by eliminating the C1-specific activity (right column in Fig. 6D) in 5 animals. The durations of the inspiratory phase of coughing before and after rostral, and caudal transections were 0.69 ± 0.04, 1.65 ± 0.3, and 0.86 ± 0.08 s, whereas the durations of the C1 phase were 0.28 ± 0.01, 0.37 ± 0.05, and 0.46 ± 0.14 s, respectively. The durations of the cough-related abdominal burst before the transection, and after rostral, and caudal transections were 0.57 ± 0.03, 0.88 ± 0.07, and 1.21 ± 0.17 s, respectively. On the other hand, the amplitudes of the phrenic nerve activity after rostral and after caudal transections were decreased to 74.0 ± 7.7 and 75.2 ± 10.6% relative to those before the transection, whereas those of the RLN activity during the C1 phase were reduced by 95.3 ± 3.8 and 74.3 ± 5.9, respectively. The amplitudes of the abdominal nerve activity during coughing were reduced by 92.0 ± 5.0 and 71.0 ± 3.7% due to the rostral and caudal transections, respectively. The phrenic nerve activity showed no significant changes in either duration or amplitude. The activity of the RLN was significantly reduced by the caudalmost transection of the RTN/pFRG (P < 0.01), albeit no statistical difference was observed regarding the duration. Meanwhile, the period of the cough-related abdominal burst was significantly prolonged and the amplitude was significantly reduced by the removal of the RTN/pFRG (P < 0.01).
SLN stimulation could induce fictive swallowing with preservation of the swallow-related RLN burst not only after the rostral but also after caudal level transection (Fig. 7). The durations of the swallow-related RLN burst before and after rostralmost and caudalmost transections were 0.40 ± 0.02, 0.42 ± 0.03, and 0.46 ± 0.04 s, respectively. The percentages of amplitudes of the RLN after rostral- and caudal-most transections compared with those before transection were 98.4 ± 5.6 and 83.0 ± 4.5, respectively. The RLN had a tendency to decrease in amplitude as a result of the caudalmost transection, although there was no significant difference between before and after the transection.
In the animals tested for only the caudalmost sectioning, the effects of the transection were the same as the results described above. The precise location of sectioning relative to surrounding regions such as the caudal tip of the RTN/pFRG and the rostral pole of the retrofacial nucleus seemed to yield uncertain results, since the cutting lines identified on the consecutive sections were likely to deteriorate due to brain tissue damage and bleeding.
DISCUSSION
The major finding of the present study is that although the activity of all types of the respiratory-related RTN/pFRG neurons was changed in synchrony with coughing and swallowing, the removal of this area had only a slight influence on the generation of these nonrespiratory behaviors. In particular, the cough-related abdominal bursting activity was preserved after the removal of RTN/pFRG neurons essential for the generation of the hypercapnia-related active expiration. These data imply that the RTN/pFRG is of critical importance for the generation of hypercapnia-induced active expiration, but it plays a minor role in the generation of coughing-induced active expiration.
The cough-related exhalation event can be divided into three phases with regard to vocal cord movement: compressive, expulsive, and narrowing phases (Korpáš and Tomori 1979). The vocal cords are adducted in the compressive phase with powerful expiratory muscle activation resulting in an abrupt rise in tracheal pressure. The vocal cords are then abducted transiently at the peak of the tracheal pressure, releasing an explosive expiratory airflow during the expulsive phase. The vocal cords are then adducted again in the narrowing phase. Considering that adductor motoneurons are activated during the compressive and narrowing phases and inhibited during the expulsive phase (Shiba et al. 1999) and that the RLN efferents consist mainly of adductor fibers, it is safe to say that the C1, C2, and C3 phases correspond to the compressive, expulsive, and narrowing phases, respectively. As an analogy, the RTN/pFRG neurons that were mainly activated during the C1 phase could be related to motor activity during the compressive phase of coughing including the glottal adduction, whereas those that strongly discharged during the C2 phase may have a close relation to the cough-associated muscle activity during the expulsive phase including cough-induced abdominal constriction.
Activities of the RTN/pFRG Neurons During Coughing and Swallowing
E-aug neurons.
E-aug neurons, which were mainly distributed in the caudal part of the RTN/pFRG, tended to fire during the expulsive phase of coughing, whereas these activities ceased during swallowing. This firing characteristic is similar to that of caudal ventral respiratory group E-aug neurons, some of which correspond to the abdominal premotor neurons (Miller et al. 1985). The RTN/pFRG is generally recognized as the central site of chemosensitivity to CO2, thereby contributing pH homeostasis by regulating respiratory control (Feldman et al. 2003; Guyenet 2008; Ritucci et al. 2005), such as by active expiration produced by the increased activity of expiratory muscle groups including the abdominal muscles in the late expiratory phase under hypercapnic conditions (Abdala et al. 2009; Iizuka and Fregosi 2007; Pagliardini et al. 2011).
Some investigators (Abdala et al. 2009; Moraes et al. 2012) have reported that an increase in the level of end-tidal CO2 facilitates activity of the RTN/pFRG E-aug neurons. Previous studies have also indicated that inhibition of GABAergic or glycinergic suppression to the RTN/pFRG region, possibly by the Bötzinger or pre-Bötzinger inhibitory neurons, increases the late expiratory activity of the RTN/pFRG neurons and thus induces active expiration (Cream et al. 2002; Morgado-Valle et al. 2010; Pagliardini et al. 2011; Rosin et al. 2006). On the other hand, the RTN/pFRG neurons project to the region of the caudal ventral respiratory group which includes abdominal muscle premotor neurons (Smith et al. 1989). In adult animals, Phox2b immunoreactive neurons in the RTN/pFRG, especially those ventromedial to the caudal end of the facial nucleus, exhibit the pronounced expiratory modulation during hypercapnia (Stornetta et al. 2006). The E-aug neurons recorded in the ventromedial portion of the RTN/pFRG may therefore include the Phox2b chemosensitive neurons. Meanwhile, the respiratory-related neurons are distributed not only in the ventromedial portion of the RTN/pFRG (Mulkey et al. 2004; Pearce et al. 1989) but also in its lateral region (Onimaru and Homma 2003). In contrast to juvenile animals, this lateral region is also related to active expiration in adult animals (Pagliardini et al. 2011). Indeed, here the E-aug neurons were broadly distributed in this area. These observations raise the prospect that the E-aug neurons in the RTN/pFRG, including Phox2b-expressing neurons, could provide the excitatory drive to the bulbospinal premotor neurons of the abdominal motoneurons, although there is no direct evidence (Abdala et al. 2009). Additional studies are needed to determine whether the abdominal premotor neurons in the ventrolateral medulla receive monosynaptic excitatory input from the RTN/pFRG E-aug neurons. In the present study, the removal of the RTN/pFRG attenuated the abdominal nerve activity during coughing as well as respiration, suggesting that the E-aug neurons in the RTN/pFRG cause active expiration via abdominal premotor neurons in the caudal ventral respiratory group not only during breathing but also during coughing (Janczewski and Feldman 2006; Miller et al. 1985; Pagliardini et al. 2011).
One concern is that the activity of respiratory neurons could be altered under hypercapnic conditions. This reconfiguration of the respiratory neuronal network under hypercapnic conditions may influence the activity of each type of respiratory neuron in the RTN/pFRG during the nonrespiratory behaviors as well as during respiration. For example, the changes in activity of the E-aug neurons during coughing may be masked under hypercapnic conditions, since these neurons are activated according to an increase in arterial CO2 during respiration (Abdala et al. 2009).
A large population of neurons in the caudal end of the RTN/pFRG was located in the area ventromedial to the facial nucleus, where the chemosensitive expiratory neurons are densely distributed (Guyenet 2008; Mulkey et al. 2004; Stornetta et al. 2006). Indeed, many E-aug neurons in this region were in synchrony with the abdominal nerve activity not only during respiration but also during coughing under hypercapnic conditions. However, we should consider the potential implications that the caudal end of the RTN/pFRG may include the rostral extended column of the Bötzinger complex.
E-dec neurons.
E-dec neurons in the RTN/pFRG were activated according to the period of the RLN activity during the compressive phase of coughing and during swallowing. The bursting activity of the RLN during the compressive phase of coughing and during swallowing tended to be attenuated after removal of the RTN/pFRG. Neurons that control the transient glottic closure during coughing and swallowing have been found in the dorsal and ventral respiratory columns in the medulla (Grélot and Bianchi 1996; Oku et al. 1994; Shiba et al. 2007; Sugiyama et al. 2011). On the other hand, the dorsolateral pons is the area where excitation or inhibition activates laryngeal postinspiratory activity (Dutschmann and Herbert 2006), and the occurrence of coughing is inhibited (Poliaček et al. 2004), and the activity of the E-dec neurons is changed in synchrony with coughing (Shannon et al. 2004a). Meanwhile, Bautista and Dutschmann (2014) pointed out that the pontine respiratory group participates in the generation of swallowing. Furthermore, there appears to be extensive interconnections among the RTN/pFRG, pontine respiratory group, and medullary respiratory group neurons (Chamberlin and Saper 1994; Dobbins and Feldman 1994; Herbert et al. 1990; Núñez-Abades et al. 1993; Rosin et al. 2006). Therefore, it seems likely that E-dec neurons in the RTN/pFRG may serve to coordinate the glottal adduction during coughing and swallowing.
I-aug neurons.
Approximately one-half of the recorded RTN/pFRG I-aug neurons were activated not only during the inspiratory but also during the expiratory phase of coughing. This paradoxical biphasic activity has not been previously observed in the other medullary inspiratory neurons (Gestreau et al. 1996; Haji et al. 2012; Oku et al. 1994; Shannon et al. 1998; Sugiyama et al. 2014). One possibility is that this expiratory activity during coughing may be responsible for disinhibition of inhibitory respiratory neurons in the Bötzinger complex (Bongianni et al. 1998; Cream et al. 2002), whose activation suppresses the activity of phrenic motoneurons, expiratory muscle premotor neurons, and laryngeal adductor motoneurons (Ezure and Manabe 1988; Ono et al. 2006). However, because the characteristics of RTN/pFRG I-aug neurons, in particular their axonal projections and transmitters, are not well understood, here we cannot discuss the details of their possible roles in coughing. During swallowing, all I-aug neurons recorded in the present study were silent as with inspiratory motoneurons of the upper airway muscles (Gestreau et al. 2000; Grélot et al. 1989; Shiba et al. 1999). On the contrary, some inspiratory neurons in the dorsal (Gestreau et al. 1996; Saito et al. 2002) and ventral respiratory group (Oku et al. 1994; Sugiyama et al. 2014) were discharged in synchrony with a weak activation of the phrenic nerve during swallowing, referred to as “swallow-breath” (Dick et al. 1993). The result that removal of the RTN/pFRG region did not affect this minor phrenic nerve activity indicates that I-aug neurons in this area are not involved in the generation of the swallow-breath. Consequently, these distinct discharge patterns of the I-aug neurons during respiration, coughing, and swallowing may be involved in the coordination of these behaviors to prevent aspiration (Pitts et al. 2013).
Phase-spanning neurons.
Many phase-spanning neurons in the RTN/pFRG were typically activated during the expiratory phase of coughing and during swallowing. Although the physiological role of the phase-spanning neurons regarding respiratory rhythm regulation have been discussed in previous studies (Connelly et al. 1990; Guyenet et al. 2005), their significance for the generation of coughing and swallowing has not been fully explored. Guyenet et al. (2005) have suggested that these phase-spanning neurons receive inhibitory inputs from either inspiratory or expiratory neurons in the medullary respiratory centers. It is therefore possible that these neurons participate in the pattern-generating processes of coughing or swallowing as do other medullary respiratory neurons (Bongianni et al. 1998; Shannon et al. 1998; Sugiyama et al. 2014). A swallow-related IE neuron orthodromically activated by the SLN was recorded in the present study, which implies that some phase-spanning neurons in the RTN/pFRG do receive laryngeal afferent information via the nucleus tractus solitarius and could act as a key center involved in coughing and swallowing (Jean 2001; Ohi et al. 2005), such that they may play a regulatory role in these behaviors. Further studies will be necessary to determine the exact role of the phase-spanning neurons involved in coughing and swallowing.
Oropharyngeal swallow is elicited by providing bolus into the pharynx, during which the physiological signals are conveyed to the nucleus tractus solitarius via the glossopharyngeal nerve as well as the SLN (Ootani et al. 1995). The activities of the RTN/pFRG neurons, a part of which receive inputs from laryngeal afferents, during swallowing elicited by electrical stimulation of the SLN might be distinct from those elicited by physiological stimuli, since the integration of afferent inputs from the upper alimentary tract could influence the elicitability of swallowing and the processing of sensory information during swallowing.
Some neurons located in the area where the facial motoneurons are distributed, may possibly include the facial motoneurons that exhibit respiratory-related activity. However, the results obtained from the collision test suggest that the neurons recorded in this area are mainly interneurons in the RTN/pFRG which project to the brain stem respiratory center (Li et al. 2004).
Influence of Brain Stem Sectioning on Respiratory and Nonrespiratory Regulations
Respiration.
The specific discharge patterns of the laryngeal and abdominal nerves during breathing were altered depending on the transection levels. The microinjection and transection studies have strongly suggested that the rostral dorsolateral pons and their descending pathways are indispensable for the generation of the postinspiratory activity of the RLN (Abdala et al. 2009; Dutschmann and Herbert 2006; Smith et al. 2007). We also showed that the postinspiratory activity is abolished by brain stem transection at the rostral level of the RTN/pFRG. Our data that the removal of the pons rostral to the RTN/pFRG, which includes the rostral dorsolateral pons, eliminated the postinspiratory RLN discharge supports this theory.
The present study showed that the removal of the RTN/pFRG region from the medulla reduces the late expiratory activation of the abdominal nerve. Although the medullary respiratory networks could vary, to some extent, in different species (Ezure et al. 1988; Richerson and Getting 1992), the functional role of the RTN/pFRG area in the regulation of respiration in guinea pigs was similar to that in the other animals previously reported (Abdala et al. 2009; Janczewski and Feldman 2006). Some authors (Abdala et al. 2009; Moraes et al. 2012; Pagliardini et al. 2011) have shown that RTN/pFRG neurons are activated in synchrony with enhanced activity of the abdominal nerve during active expiration. Several lines of evidence such as the above-mentioned results have indicated that the RTN/pFRG area is essential for the generation of active expiration under hypercapnic conditions (Abdala et al. 2009; Janczewski and Feldman 2006; Pagliardini et al. 2011; Ritucci et al. 2005; Sato et al. 1992). This theory can explain the etiology of a congenital functional disorder of central chemoreception; patients with congenital central hypoventilation syndrome, in whom the functional impairment of the RTN/pFRG develops, suffer from fatal respiratory failure attributed to insusceptibility to hypercapnia (Dubreuil et al. 2008). On the other hand, the neuronal circuitry between the Bötzinger complex and pre-Bötzinger complex may also be responsible for the generation of the late expiratory activity in the neonatal period (Molkov et al. 2010; Smith et al. 2007). However, the influence of this area on the regulation of active expiration is attenuated in the growth process, and eventually disappears in the adult (Pagliardini et al. 2011).
The removal of the RTN/pFRG markedly reduced the respiratory rate, and in particular prolonged the expiratory phase, providing supportive evidence that the RTN/pFRG regulates respiratory rhythm, especially expiratory duration (Janczewski and Feldman 2006; Onimaru and Homma 2003). The changes in the respiratory rate due to the rostralmost transection tended to be smaller than those shown by Abdala et al. (2009), possibly because vagal intact animals, in which the pulmonary afferent inputs could affect the respiratory rhythm, were used in the present study (Bianchi and Gestreau 2009). Furthermore, the influence of age on the respiratory rhythm regulation should be considered when interpreting the effects of the transections. In juvenile animals, the pre-I neurons in the RTN/pFRG act as a respiratory pacemaker; however, in adult animals this specific activity is not present, which supports the view that juvenile and adult animals have distinct regulatory mechanisms regarding respiratory rhythmogenesis (Guyenet et al. 2005).
Coughing.
The parabrachial and Kölliker-Fuse nuclei, a part of the pontine respiratory group, have been suggested to contribute to the generation of coughing (Gestreau et al. 1997; Shannon et al. 2004b). Poliaček et al. (2004) have reported that bilateral chemical lesions of the rostral dorsolateral pons suppress the cough reflex evoked by mechanical stimulation of the laryngotracheal mucosa. Conversely, the present study showed that, regardless of the removal of the pons rostral to the RTN/pFRG, coughing can be evoked by stimulation of the laryngeal afferent nerve or the tracheal mucosa, and the sequential pattern of cough-related nerve activities is preserved. One possibility is that this difference in evoking coughing may be attributed to the species differences. Although the influence of rostralmost sectioning on the respiratory-related nerve activity was similar to that in other animals (Abdala et al. 2009; Smith et al. 2007), the cough-generating neuronal networks including the pontine region are probably distinct between species. The other possibility is that the elimination of the descending inputs from higher centers by the transverse brain stem sectioning affected the sensitivity of the cough reflex. Since the medullary neuronal circuits involved in cough generation can be suppressed by the inhibition of the pontine respiratory group, this region is certainly important for evoking coughing (Poliaček et al. 2004). Our findings raise the possibility that the sensitivity of the cough reflex could be suppressed from higher centers (Widdicombe 1995). This is physiologically appropriate since, when the bolus passes over the larynx during swallowing, an inappropriate cough reflex may interfere with bolus transit. Consequently, our data indicate that the parabrachial and Kölliker-Fuse nuclei are not a source of the RLN activity during the compressive phase of coughing, although the neurons in this region participate in the cough-generating neuronal network (Gestreau et al. 1997; Jakuš et al. 2008).
As discussed above, the E-aug neurons in the RTN/pFRG are thought to be necessary for the generation of the late expiratory activity during hypercapnia (Pagliardini et al. 2011). In the present study, the E-aug neurons recorded under hypercapnic conditions were broadly distributed in this area, and tended to be activated according to the cough-related abdominal burst. Thus it is also feasible that the slight attenuation of the cough-related abdominal activity, following the caudalmost transection, could be due to the removal of the RTN/pFRG E-aug neurons. However, in contrast to the hypercapnia-induced abdominal activation, the cough-related abdominal bursting activity was not largely affected by the caudalmost RTN/pFRG transection. This finding implies that the excitatory drive to the abdominal motoneuron pool during coughing is generated within the brain stem circuitry caudal to the RTN/pFRG.
The phenomenon of the cough-related abdominal activity which started before cessation of the phrenic activity occurred sporadically during the series of recording sessions (see the middle right column of Fig. 6D as an example). This is not unexpected, since the activity of the abdominal muscles during the inspiratory phase of coughing can possibly be observed (Bolser et al. 2000).
The effects of caudal level transection on the cough-related RLN varied from animal to animal; the transection did not change the activity pattern in 3 animals but sometimes altered the activity from the bimodal discharge pattern to the single peak pattern in 5 animals. A caveat should be considered when interpreting these results. Slight pulsation of the brain stem due to the arterial pulsation may be attributed to the brain damage in the immediate vicinity of the cutting blade positioned in contrast to the perfused model. It thus seems likely that the variations of the effects of transection were attributed to variations of spread of brain damage induced by mechanical section, because the Bötzinger complex, which is located adjacent to the RTN/pFRG, is a strong candidate site for the generation of the compressive phase (Shiba et al. 2007). This may be due to the elimination of the compressive phase as observed in the present study.
Swallowing.
The medullary swallowing center generates sequential rhythmic movements of the upper aerodigestive tract (Jean 2001; Kessler and Jean 1985; Sugiyama et al. 2011; Umezaki et al. 1998a). The present study showed that, although the activity in the majority of RTN/pFRG neurons is changed in synchrony with swallowing, the patterned motor activity of swallowing remains unchanged after the removal of these RTN/pFRG neurons. While the spatiotemporal activity patterns of the upper airway and alimentary tract muscles involved in swallowing are always constant (Miller 1972; Umezaki et al. 1998b), the magnitude of muscular contractions could be altered by changes in the bolus volume to support its transport (Perlman et al. 1999). Some investigators have proposed that a subset of neurons in the pons functions as a relay station conveying laryngeal afferent inputs to higher brain centers, such that these neurons contribute to the regulation of swallowing (Jean et al. 1975; Perlman et al. 1999). The RTN/pFRG neurons involved in swallowing may participate in the sensory feedback regulation of swallowing, since some of them receive sensory inputs from the larynx (see Fig. 4C).
The motor patterns of the other nerves, such as the hypoglossal nerve, may be needed for a more detailed analysis of the swallow-related motor activities due to the brain stem transections (Jean 2001), although fictive swallowing induced by electrical stimulation of the SLN can be identified by the specific bursting activity of the RLN (Umezaki et al. 1998b). Our data would also suggest that there may be other influences, caused by the caudalmost sectioning, on the swallowing behavior, including the activities of the myohyoid, geniohyoid, and thyrohyoid muscles innervated by the hypoglossal nerve.
Perspectives and Significance
The present findings raise the prospect that although various types of respiratory-related neurons in the RTN/pFRG exhibit highly synchronous activity during the nonrespiratory behaviors including coughing and swallowing, these neurons are not a critical element in the pattern generation of these behaviors. The RTN/pFRG contributes to maintaining homeostasis by regulating respiratory rhythm and forced expiratory effort in response to acidemia (Feldman et al. 2003; Guyenet 2008; Onimaru and Homma 2003; Ritucci et al. 2005). Likewise, the data provided in this study suggest that these neurons may also participate in the feedback system involved in coughing and swallowing, as well as respiration to achieve the appropriate movements, although there are no previous studies to support this hypothesis. Future studies are necessary to reveal the functional contributions of the ascending or descending inputs from different sites of the brain stem, possibly including the central pattern generators for those behaviors, as such studies may provide insight into the highly sophisticated optimization of sequential movements of respiratory muscles during the airway protective reflexes.
GRANTS
This work was supported by Grant-in-Aid for Young Scientists (B) Grant No. 25861581 and Scientific Research (B) Grant No. 25293350.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.S. and K.S. conception and design of research; Y.S. and S.M. performed experiments; Y.S. and K.S. analyzed data; Y.S., K.S., and T.U. interpreted results of experiments; Y.S. prepared figures; Y.S. drafted manuscript; Y.S. and K.S. edited and revised manuscript; Y.S., K.S., S.M., T.U., H.S., and Y.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. T. Suzuki for comments on an earlier version of the manuscript.
Glossary
- ABD
Abdominal nerve
- E
Expiratory phase
- E-aug
Expiratory neurons with an augmenting firing pattern
- E-dec
Expiratory neurons with a decrementing firing pattern
- EI
Expiratory-inspiratory phase-spanning neurons
- I
Inspiratory phase
- I-aug
Inspiratory neurons with an augmenting firing pattern
- IE
Inspiratory-expiratory phase-spanning neurons
- Inst Freq
Instantaneous frequency
- ML
Lateral to the midline
- NA
Nucleus ambiguus
- PH
Nucleus prepositus hypoglossi
- PHR
Phrenic nerve
- PON
Preolivary nucleus
- PPR
Postpyramidal nucleus of the raphe
- RB
Restiform body
- RLN
Recurrent laryngeal nerve
- RLN stim
Stimulation of the RLN
- RTN/pFRG
Retrotrapezoid/parafacial respiratory group
- SLN
Superior laryngeal nerve
- SLN stim
Stimulation of the SLN
- SO
Superior olive
- SOM
Medial nucleus of the superior olive
- Trachea stim
Stimulation of the tracheal mucosa
- VLD
Lateral vestibular nucleus, dorsal division
- 5SP
Spinal trigeminal nucleus
- 5ST
Spinal trigeminal tract
- 7N
Facial nucleus
REFERENCES
- Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol 587: 3539–3559, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arata A, Onimaru H, Homma I. The adrenergic modulation of firings of respiratory rhythm-generating neurons in medulla-spinal cord preparation from newborn rat. Exp Brain Res 119: 399–408, 1998. [DOI] [PubMed] [Google Scholar]
- Bautista TG, Dutschmann M. Ponto-medullary nuclei involved in the generation of sequential pharyngeal swallowing and concomitant protective laryngeal adduction in situ. J Physiol 592: 2605–2623, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi AL, Gestreau C. The brainstem respiratory network: an overview of a half century of research. Respir Physiol Neurobiol 168: 4–12, 2009. [DOI] [PubMed] [Google Scholar]
- Bolser D. Fictive cough in the cat. J Appl Physiol 71: 2325–2331, 1991. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Reier PJ, Davenport PW. Responses of the anterolateral abdominal muscles during cough and expiratory threshold loading in the cat. J Appl Physiol 88: 1207–1214, 2000. [DOI] [PubMed] [Google Scholar]
- Bongianni F, Mutolo D, Fontana GA, Pantaleo T. Discharge patterns of Bötzinger complex neurons during cough in the cat. Am J Physiol Regul Integr Comp Physiol 274: R1015–R1024, 1998. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J Neurosci 14: 6500–6510, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Are there serotonergic projections from raphe and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci Lett 105: 34–40, 1989. [DOI] [PubMed] [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Respiratory activity in retrotrapezoid nucleus in cat. Am J Physiol Lung Cell Mol Physiol 258: L33–L44, 1990. [DOI] [PubMed] [Google Scholar]
- Cream C, Li A, Nattie E. The retrotrapezoid nucleus (RTN): local cytoarchitecture and afferent connections. Respir Physiol Neurobiol 130: 121–137, 2002. [DOI] [PubMed] [Google Scholar]
- Dick TE, Oku Y, Romaniuk JR, Cherniack NS. Interaction between central pattern generators for breathing and swallowing in the cat. J Physiol 465: 715–730, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347: 64–86, 1994. [DOI] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci USA 105: 1067–1072, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The Kolliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci 24: 1071–1084, 2006. [DOI] [PubMed] [Google Scholar]
- Ezure K, Manabe M, Yamada H. Distribution of medullary respiratory neurons in the rat. Brain Res 455: 262–270, 1988. [DOI] [PubMed] [Google Scholar]
- Ezure K, Manabe M. Decrementing expiratory neurons of the Bötzinger complex. II. Direct inhibitory synaptic linkage with ventral respiratory group neurons. Exp Brain Res 72: 159–166, 1988. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestreau C, Bianchi AL, Grélot L. Differential brainstem fos-like immunoreactivity after laryngeal-induced coughing and its reduction by codeine. J Neurosci 17: 9340–9352, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestreau C, Grélot L, Bianchi AL. Activity of respiratory laryngeal motoneurons during fictive coughing and swallowing. Exp Brain Res 130: 27–34, 2000. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Milano S, Bianchi AL, Grélot L. Activity of dorsal respiratory group inspiratory neurons during laryngeal-induced fictive coughing and swallowing in decerebrate cats. Exp Brain Res 108: 247–256, 1996. [DOI] [PubMed] [Google Scholar]
- Grélot L, Barillot JC, Bianchi AL. Pharyngeal motoneurones: respiratory-related activity and responses to laryngeal afferents in the decerebrate cat. Exp Brain Res 78: 336–344, 1989. [DOI] [PubMed] [Google Scholar]
- Grélot L, Bianchi AL. Multifunctional medullary respiratory neurons. In: Neural Control of the Respiratory Muscles, edited by Miller AD, Bianchi AL, Bishop BP. Boca Raton, FL: CRC, 1996, p. 297–304. [Google Scholar]
- Grélot L, Milano S. Diaphragmatic and abdominal muscle activity during coughing in the decerebrate cat. Neuroreport 2: 165–168, 1991. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci 25: 8938–47, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol 105: 404–416, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji A, Ohi Y, Kimura S. Cough-related neurons in the nucleus tractus solitarius of decerebrate cats. Neuroscience 218: 100–109, 2012. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990. [DOI] [PubMed] [Google Scholar]
- Iizuka M, Fregosi RF. Influence of hypercapnic acidosis and hypoxia on abdominal expiratory nerve activity in the rat. Respir Physiol Neurobiol 157: 196–205, 2007. [DOI] [PubMed] [Google Scholar]
- Jakuš J, Poliaček I, Halašová E, Murin P, Knocikova J, Tomori Z, Bolser DC. Brainstem circuitry of tracheal-bronchial cough: c-fos study in anesthetized cats. Respir Physiol Neurobiol 160: 289–300, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570: 407–420, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A, Car A, Roman C. Comparison of activity in pontine versus medullary neurones during swallowing. Exp Brain Res 22: 211–220, 1975. [DOI] [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 81: 929–969, 2001. [DOI] [PubMed] [Google Scholar]
- Kessler JP, Jean A. Identification of the medullary swallowing regions in the rat. Exp Brain Res 57: 256–263, 1985. [DOI] [PubMed] [Google Scholar]
- Kessler JP, Jean A. Inhibitory influence of monoamines and brainstem monoaminergic regions on the medullary swallowing reflex. Neurosci Lett 65: 41–46, 1986. [DOI] [PubMed] [Google Scholar]
- Korpáš J, Tomori Z. Cough and Other Respiratory Reflexes. Basel: Karger, 1979. [Google Scholar]
- Li C, Guan Z, Chan Y, Zheng Y. Projections from facial nucleus interneurons to the respiratory groups of brainstem in the rat. Neurosci Lett 368: 25–28, 2004. [DOI] [PubMed] [Google Scholar]
- Lois JH, Rice CD, Yates BJ. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol 106: 138–152, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AD, Ezure K, Suzuki I. Control of abdominal muscles by brain stem respiratory neurons in the cat. J Neurophysiol 54: 155–167, 1985. [DOI] [PubMed] [Google Scholar]
- Miller AJ. Significance of sensory inflow to the swallowing reflex. Brain Res 43: 147–159, 1972. [DOI] [PubMed] [Google Scholar]
- Molkov YI, Abdala AP, Bacak BJ, Smith JC, Paton JF, Rybak IA. Late-expiratory activity: emergence and interactions with the respiratory CpG. J Neurophysiol 104: 2713–2729, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes DJ, Dias MB, Cavalcanti-Kwiatkoski R, Machado BH, Zoccal DB. Contribution of the retrotrapezoid nucleus/parafacial respiratory region to the expiratory-sympathetic coupling in response to peripheral chemoreflex in rats. J Neurophysiol 108: 882–890, 2012. [DOI] [PubMed] [Google Scholar]
- Morgado-Valle C, Baca SM, Feldman JL. Glycinergic pacemaker neurons in preBötzinger complex of neonatal mouse. J Neurosci 30: 3634–3639, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004. [DOI] [PubMed] [Google Scholar]
- Nishino T, Honda Y, Kohchi T, Shirahata M, Yonezawa T. Effects of increasing depth of anaesthesia on phrenic nerve and hypoglossal nerve activity during the swallowing reflex in cats. Br J Anaesth 57: 208–213, 1985. [DOI] [PubMed] [Google Scholar]
- Núñez-Abades PA, Morillo AM, Pásaro R, Nfifiez-abades PA, Rosario P. Brainstem connections of the rat ventral respiratory subgroups: afferent projections. J Auton Nerv Syst 42: 99–118, 1993. [DOI] [PubMed] [Google Scholar]
- Ohi Y, Yamazaki H, Takeda R, Haji A. Functional and morphological organization of the nucleus tractus solitarius in the fictive cough reflex of guinea pigs. Neurosci Res 53: 201–209, 2005. [DOI] [PubMed] [Google Scholar]
- Oku Y, Tanaka I, Ezure K. Activity of bulbar respiratory neurons during fictive coughing and swallowing in the decerebrate cat. J Physiol 480: 309–324, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci 23: 1478–1486, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Shiba K, Nakazawa K, Shimoyama I. Synaptic origin of the respiratory-modulated activity of laryngeal motoneurons. Neuroscience 140: 1079–1088, 2006. [DOI] [PubMed] [Google Scholar]
- Ootani S, Umezaki T, Shin T, Murata Y. Convergence of afferents from the SLN and GPN in cat medullary swallowing neurons. Brain Res Bull 37: 397–404, 1995. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci 31: 2895–2905, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce RA, Stornetta RL, Guyenet PG. Retrotrapezoid nucleus in the rat. Neurosci Lett 101: 138–142, 1989. [DOI] [PubMed] [Google Scholar]
- Perlman AL, Palmer PM, McCulloch TM, Vandaele DJ. Electromyographic activity from human laryngeal, pharyngeal, and submental muscles during swallowing. J Appl Physiol 86: 1663–1669, 1999. [DOI] [PubMed] [Google Scholar]
- Pitts T, Rose MJ, Mortensen AN, Poliacek I, Sapienza CM, Lindsey BG, Morris KF, Davenport PW, Bolser DC. Coordination of cough and swallow: a meta-behavioral response to aspiration. Respir Physiol Neurobiol 189: 543–551, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliaček I, Jakuš J, Stránsky A, Baráni H, Halašová E, Tomori Z. Cough, expiration and aspiration reflexes following kainic acid lesions to the pontine respiratory group in anesthetized cats. Physiol Res 53: 155–163, 2004. [PubMed] [Google Scholar]
- Richerson GB, Getting PA. Medullary respiratory neurons in the guinea pig: localization and firing patterns. Brain Res 591: 79–87, 1992. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol Regul Integr Comp Physiol 289: R851–R861, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 499: 64–89, 2006. [DOI] [PubMed] [Google Scholar]
- Saito Y, Tanaka I, Ezure K. Morphology of the decrementing expiratory neurons in the brainstem of the rat. Neurosci Res 44: 141–153, 2002. [DOI] [PubMed] [Google Scholar]
- Sato M, Severinghaus JW, Basbaum AI. Medullary CO2 chemoreceptor neuron identification by c-fos immunocytochemistry. J Appl Physiol 73: 96–100, 1992. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol 84: 2020–2035, 1998. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. Pontine respiratory group neuron discharge is altered during fictive cough in the decerebrate cat. Respir Physiol Neurobiol 142: 43–54, 2004a. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. Production of reflex cough by brainstem respiratory networks. Pulm Pharmacol Ther 17: 369–376, 2004b. [DOI] [PubMed] [Google Scholar]
- Shiba K, Nakazawa K, Ono K, Umezaki T. Multifunctional laryngeal premotor neurons: their activities during breathing, coughing, sneezing, and swallowing. J Neurosci 27: 5156–5162, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K, Satoh I, Kobayashi N, Hayashi F. Multifunctional laryngeal motoneurons: an intracellular study in the cat. J Neurosci 19: 2717–2727, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol 281: 69–96, 1989. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26: 10305–14, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Shiba K, Mukudai S, Umezaki T, Hisa Y. Activity of respiratory neurons in the rostral medulla during vocalization, swallowing, and coughing in guinea pigs. Neurosci Res 80: 17–31, 2014. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Shiba K, Nakazawa K, Suzuki T, Umezaki T, Ezure K, Abo N, Yoshihara T, Hisa Y. Axonal projections of medullary swallowing neurons in guinea pigs. J Comp Neurol 519: 2193–2211, 2011. [DOI] [PubMed] [Google Scholar]
- Umezaki T, Matsuse T, Shin T. Medullary swallowing-related neurons in the anesthetized cat. Neuroreport 9: 1793–1798, 1998a. [DOI] [PubMed] [Google Scholar]
- Umezaki T, Shiba K, Zheng Y, Miller AD. Upper airway motor outputs during vomiting versus swallowing in the decerebrate cat. Brain Res 781: 25–36, 1998b. [DOI] [PubMed] [Google Scholar]
- Viemari JC. Noradrenergic modulation of the respiratory neural network. Respir Physiol Neurobiol 164: 123–130, 2008. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. Neurophysiology of the cough reflex. Eur Respir J 8: 1193–1202, 1995. [DOI] [PubMed] [Google Scholar]
- Yamanishi T, Takao K, Koizumi H, Ishihama K, Nohara K, Komaki M, Enomoto A, Yokota Y, Kogo M. Alpha2-adrenoceptors coordinate swallowing and respiration. J Dent Res 89: 258–263, 2010. [DOI] [PubMed] [Google Scholar]