Figure 3.
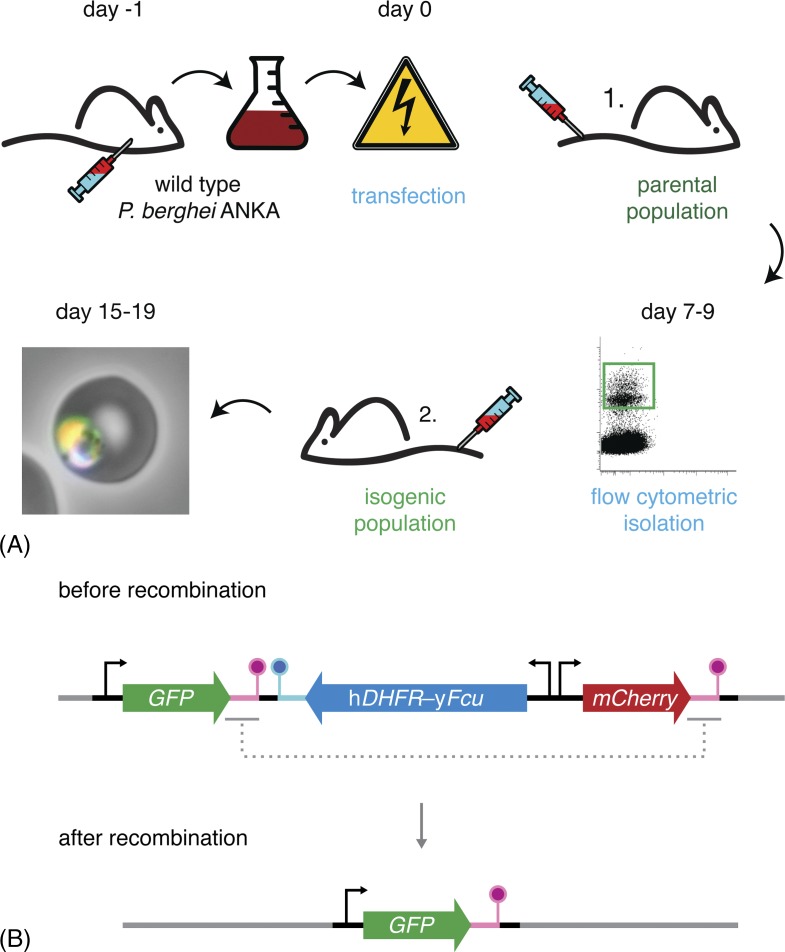
Flow cytometry-based methods reduce workload and animal usage. (A) Schematic representation of the P. berghei transfection and mutant isolation protocol. Blood of a mouse infected with wild-type parasites is harvested by cardiac puncture and cultured overnight. Following maturation, the parasites fail to egress and arrest at the schizont stage. These schizonts are purified and transfected with the targetting constructs. Transfected merozoites are injected intravenously into a naïve mouse. Administration of pyrimethamine in the drinking water favours the growth of successfully modified parasites, which now also express a fluorescent protein. When the parasitaemia is 0.1–1.0% (typically 7–9 days after transfection), 50 mutant parasites are isolated by flow cytometry and transferred to a naïve mouse. 8–10 days after injection, the isogenic parasite line can be harvested, stored or transferred and tested. (B) Schematic representation of the ‘gene-out, marker-out’ strategy exemplifying recycling of the hDHFR-yFcu drug-selectable cassette.68 Successfully transfected and isolated parasites harbour a fluorescent cassette (GFP; green), a drug-selectable cassette (hDHFR-yFcu; blue), and a second fluorescent cassette (mCherry; red). After intravenous injection into a naïve mouse, 5-fluorocytosine (5-FC) is administered in the drinking water. This favours the growth of parasites that have successfully lost yFcu by homologous recombination of the duplicated sequences flanking the drug-selectable cassette and the mCherry marker (magenta). Finally, recycled parasites can be isolated using flow cytometry through the selection of GFP-positive, mCherry-negative parasites.