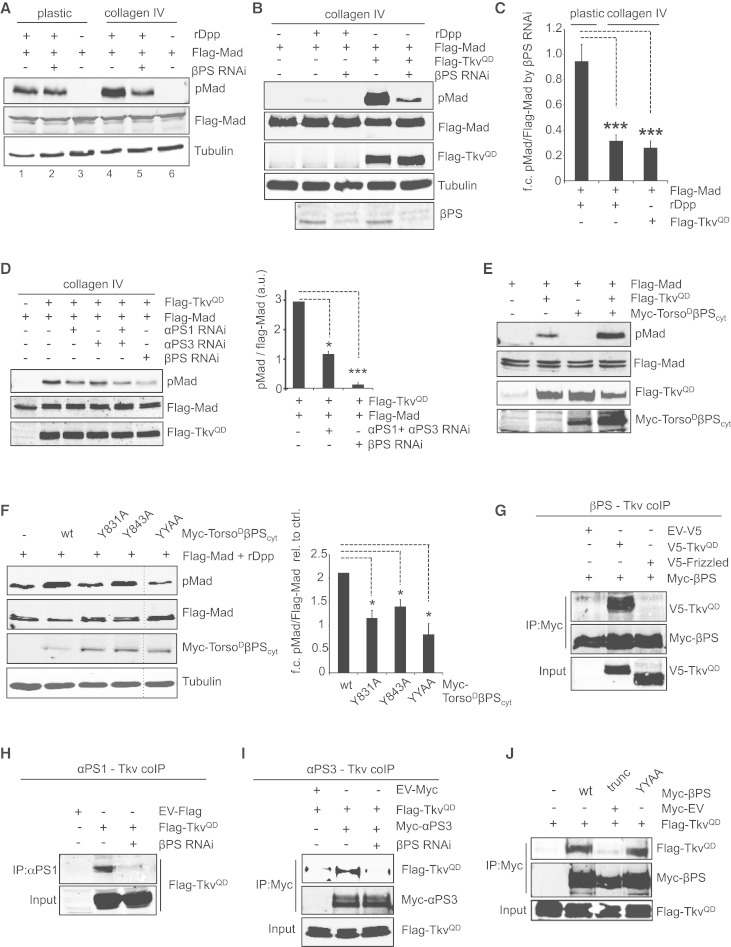
Figure 4.
Molecular Mechanism of Integrin-Signaling-Mediated Enhancement of the BMP Pathway
(A) Western blot for Flag-Mad, pMad, and tubulin in S2R+ cells transfected with Flag-Mad, which were plated on either plastic or collagen IV and treated with rDpp and βPS RNAi, as indicated.
(B) Western blot for Flag-Mad, pMad, Flag-TkvQD, βPS, and tubulin in cells transfected with Flag-Mad and plated on collagen IV. BMP signaling was activated by co-transfection of Flag-TkvQD or treatment with rDpp, and cells were treated with βPS RNAi as indicated.
(C) Quantification of the experiments in (A) and (B), showing fold reduction in pMad activation by βPS RNAi on plastic- or collagen-IV-plated cells. pMad levels were normalized to total Mad (Flag-Mad) in each sample. n = 3–6; ∗∗∗p < 0.001 (one-way ANOVA).
(D) Western blot as described in (B) with quantitation, but cells were also treated with αPS1 and/or αPS3 RNAi.
(E) Western blot for Flag-Mad, pMad, Flag-TkvQD, and Myc-TorsoDβPScyt in cells transfected with Flag-Mad, Flag-TkvQD, and Myc-TorsoDβPScyt and plated on plastic.
(F) As in (E), except cells were transfected with Flag-Mad and Myc-tagged wild-type or mutant TorsoDβPScyt, and treated with rDpp. Quantitation shows increase in pMad (n = 4); ∗p < 0.05, ∗∗p < 0.001 (paired t tests).
(G–I) Co-immunoprecipitation experiments between Flag-TkvQD and the βPS (G), αPS1 (H), and αPS3 (I) integrin subunits. Frizzled is a negative control.
(J) Co-immunoprecipitation between Flag-TkvQD and Myc-βPS forms with truncation of the cytoplasmic tail or both NPXY motifs mutated.
All error bars show SEM. See also Figure S4.